Paper Menu >>
Journal Menu >>
 Journal of Minerals & Materials Characterization & Engineering, Vol. 9, No.11, pp.973-983, 2010 jmmce.org Printed in the USA. All rights reserved 973 Beneficiation of Synthetic Iron Ore Kaolinite Mixture Using Selective Flocculation Lopamudra Pandaa*, S.K. Biswalb, Vilas Tathavadkara a R&D, Tata Steel Limited, Jamshedpur, Jharkhand, India. b Institute of Minerals and Materials Technology, Council of Scientific and Industrial Research, Bhubaneswar, Orissa, India. *Corresponding Author: l.panda@tatasteel.com ABSTRACT High demand of steel necessitates the use of low and lean grade iron ores by beneficiating them. However the fines, slimes and tailings generated in the process of beneficiating these low grade iron ores contains good amount of iron values. These iron values can be recovered by further beneficiating the fines, slimes and tailing by selective flocculation, which is defined as the selective agglomeration of iron ore particles using flocculant. In the present study an attempt has been taken to study the applicability of selective flocculation to Indian iron ore using corn starch as flocculant. A detail mineralogical and chemical characterization of iron ore and kaolinite were performed; in addition to this the flocculant was characterized by FTIR which gives an idea about the functional groups present in the flocculant. Different experiments were performed to optimize the operating parameters of the selective flocculation process. It was observed that product grade and recovery was strongly affected by pH of slurry and solid concentration compared to the flocculant dose. It was also found that the iron value can be upgraded to 63-66.9 % from the feed iron value of 37.65-55.33 % with the recovery of 63-90 %. Keywords: Selective flocculation; flocculant; iron ore; starch. 1. INTRODUCTION Steel plays a vital role in growth of any country and especially for developing country like India demand of steel is ever increasing. This high demand for steel forces the use of low grade iron ore. These iron ores contain alumina and silica as gangue material along with iron mineral. If the alumina content increases with iron mineral, the viscosity of slag increases which leads to consumption of the fuel at high rate; and decreases the productivity of the process. Because of which these iron ores needs to be beneficiated before use. However beneficiation of these low 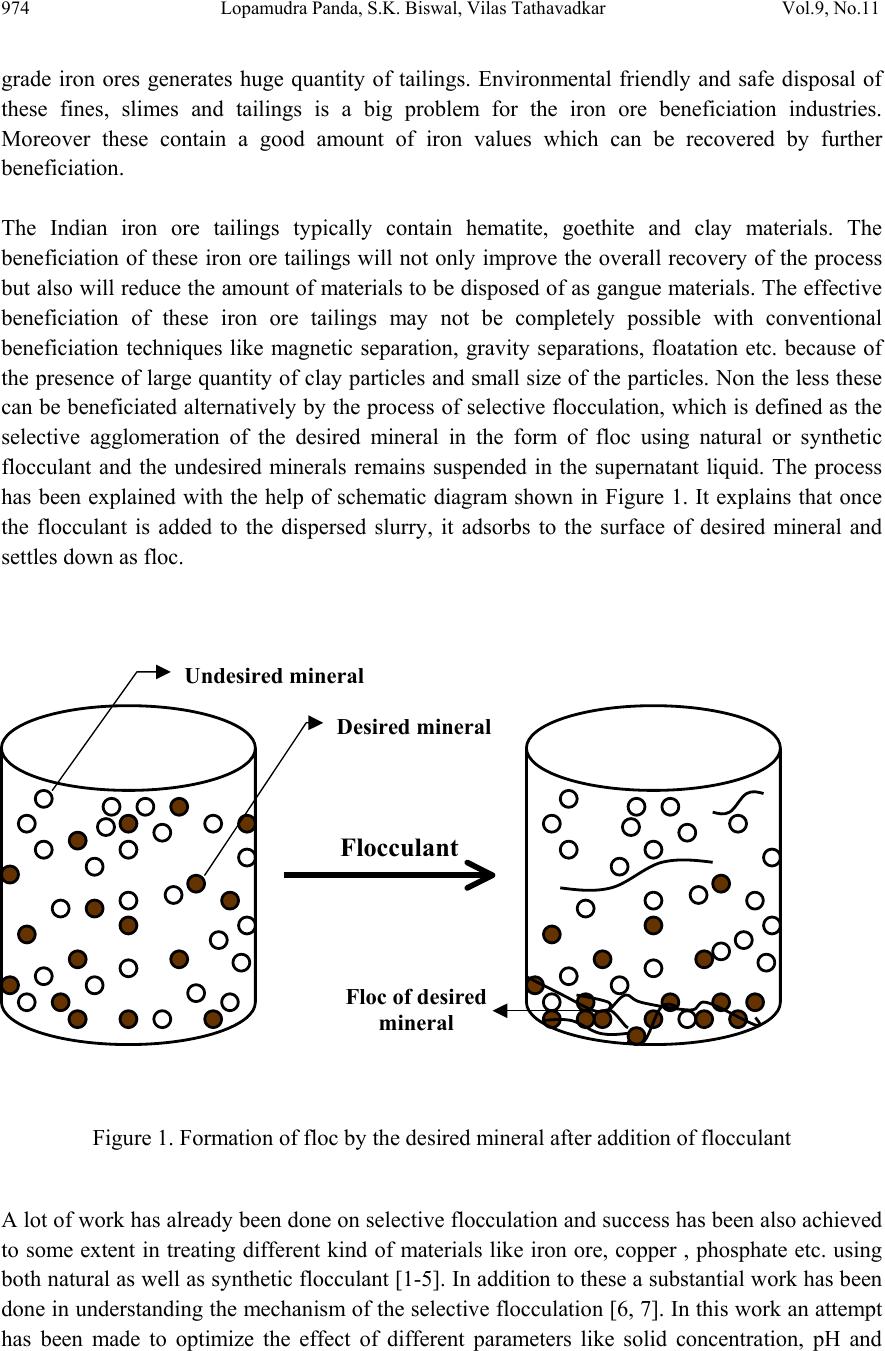 974 Lopamudra Panda , S.K. Biswal, V i l a s T athavadkar Vol.9, No.11 grade iron ores generates huge quantity of tailings. Environmental friendly and safe disposal of these fines, slimes and tailings is a big problem for the iron ore beneficiation industries. Moreover these contain a good amount of iron values which can be recovered by further beneficiation. The Indian iron ore tailings typically contain hematite, goethite and clay materials. The beneficiation of these iron ore tailings will not only improve the overall recovery of the process but also will reduce the amount of materials to be disposed of as gangue materials. The effective beneficiation of these iron ore tailings may not be completely possible with conventional beneficiation techniques like magnetic separation, gravity separations, floatation etc. because of the presence of large quantity of clay particles and small size of the particles. Non the less these can be beneficiated alternatively by the process of selective flocculation, which is defined as the selective agglomeration of the desired mineral in the form of floc using natural or synthetic flocculant and the undesired minerals remains suspended in the supernatant liquid. The process has been explained with the help of schematic diagram shown in Figure 1. It explains that once the flocculant is added to the dispersed slurry, it adsorbs to the surface of desired mineral and settles down as floc. Figure 1. Formation of floc by the desired mineral after addition of flocculant A lot of work has already been done on selective flocculation and success has been also achieved to some extent in treating different kind of materials like iron ore, copper , phosphate etc. using both natural as well as synthetic flocculant [1-5]. In addition to these a substantial work has been done in understanding the mechanism of the selective flocculation [6, 7]. In this work an attempt has been made to optimize the effect of different parameters like solid concentration, pH and Flocculant Undesired mineral Desired mineral Floc of desired mineral 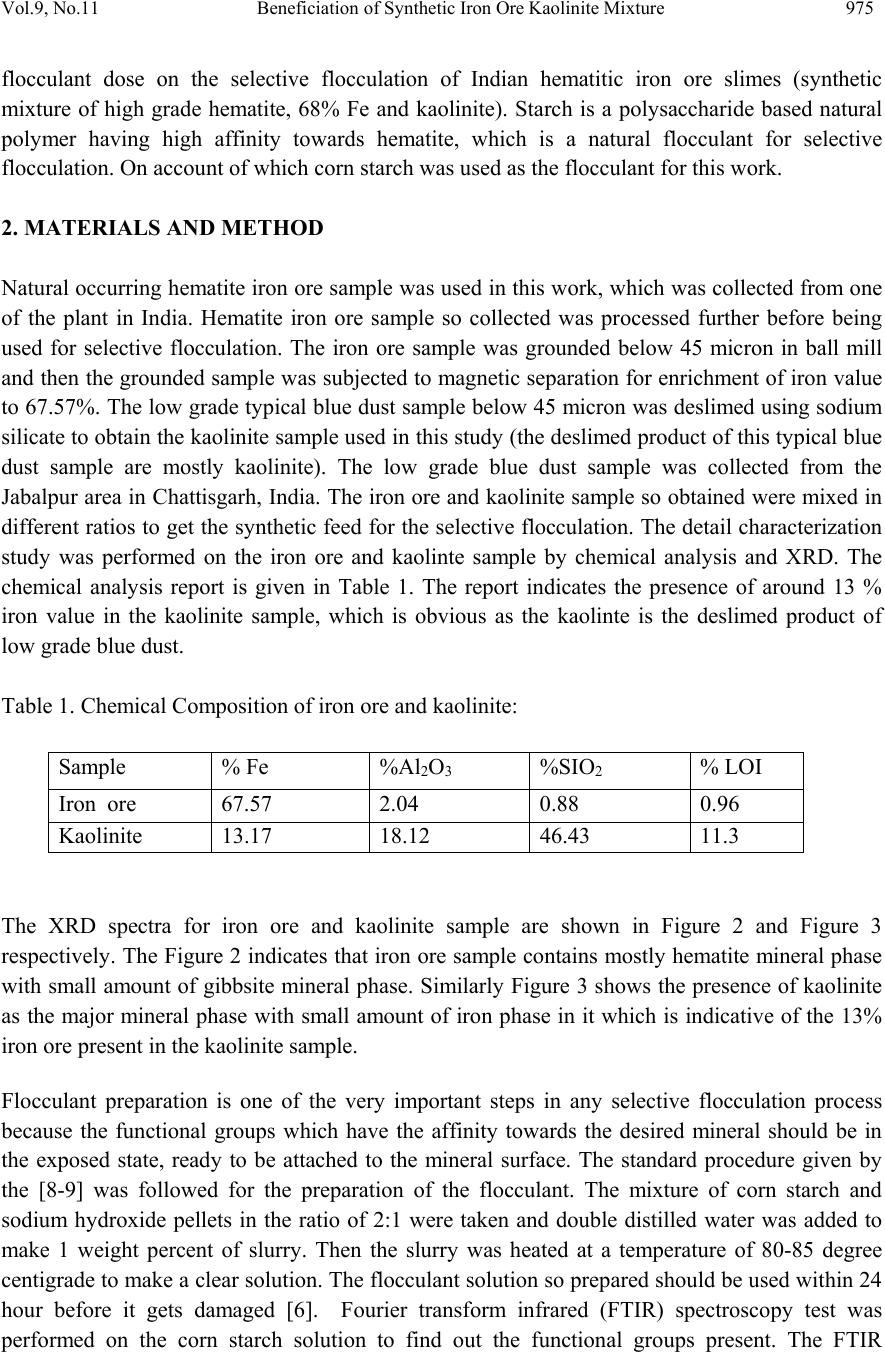 Vol.9, No.11 Beneficiation of Synthetic Iron Ore Kaolinite Mixture 975 flocculant dose on the selective flocculation of Indian hematitic iron ore slimes (synthetic mixture of high grade hematite, 68% Fe and kaolinite). Starch is a polysaccharide based natural polymer having high affinity towards hematite, which is a natural flocculant for selective flocculation. On account of which corn starch was used as the flocculant for this work. 2. MATERIALS AND METHOD Natural occurring hematite iron ore sample was used in this work, which was collected from one of the plant in India. Hematite iron ore sample so collected was processed further before being used for selective flocculation. The iron ore sample was grounded below 45 micron in ball mill and then the grounded sample was subjected to magnetic separation for enrichment of iron value to 67.57%. The low grade typical blue dust sample below 45 micron was deslimed using sodium silicate to obtain the kaolinite sample used in this study (the deslimed product of this typical blue dust sample are mostly kaolinite). The low grade blue dust sample was collected from the Jabalpur area in Chattisgarh, India. The iron ore and kaolinite sample so obtained were mixed in different ratios to get the synthetic feed for the selective flocculation. The detail characterization study was performed on the iron ore and kaolinte sample by chemical analysis and XRD. The chemical analysis report is given in Table 1. The report indicates the presence of around 13 % iron value in the kaolinite sample, which is obvious as the kaolinte is the deslimed product of low grade blue dust. Table 1. Chemical Composition of iron ore and kaolinite: Sample % Fe %Al2O3 %SIO2 % LOI Iron ore 67.57 2.04 0.88 0.96 Kaolinite 13.17 18.12 46.43 11.3 The XRD spectra for iron ore and kaolinite sample are shown in Figure 2 and Figure 3 respectively. The Figure 2 indicates that iron ore sample contains mostly hematite mineral phase with small amount of gibbsite mineral phase. Similarly Figure 3 shows the presence of kaolinite as the major mineral phase with small amount of iron phase in it which is indicative of the 13% iron ore present in the kaolinite sample. Flocculant preparation is one of the very important steps in any selective flocculation process because the functional groups which have the affinity towards the desired mineral should be in the exposed state, ready to be attached to the mineral surface. The standard procedure given by the [8-9] was followed for the preparation of the flocculant. The mixture of corn starch and sodium hydroxide pellets in the ratio of 2:1 were taken and double distilled water was added to make 1 weight percent of slurry. Then the slurry was heated at a temperature of 80-85 degree centigrade to make a clear solution. The flocculant solution so prepared should be used within 24 hour before it gets damaged [6]. Fourier transform infrared (FTIR) spectroscopy test was performed on the corn starch solution to find out the functional groups present. The FTIR 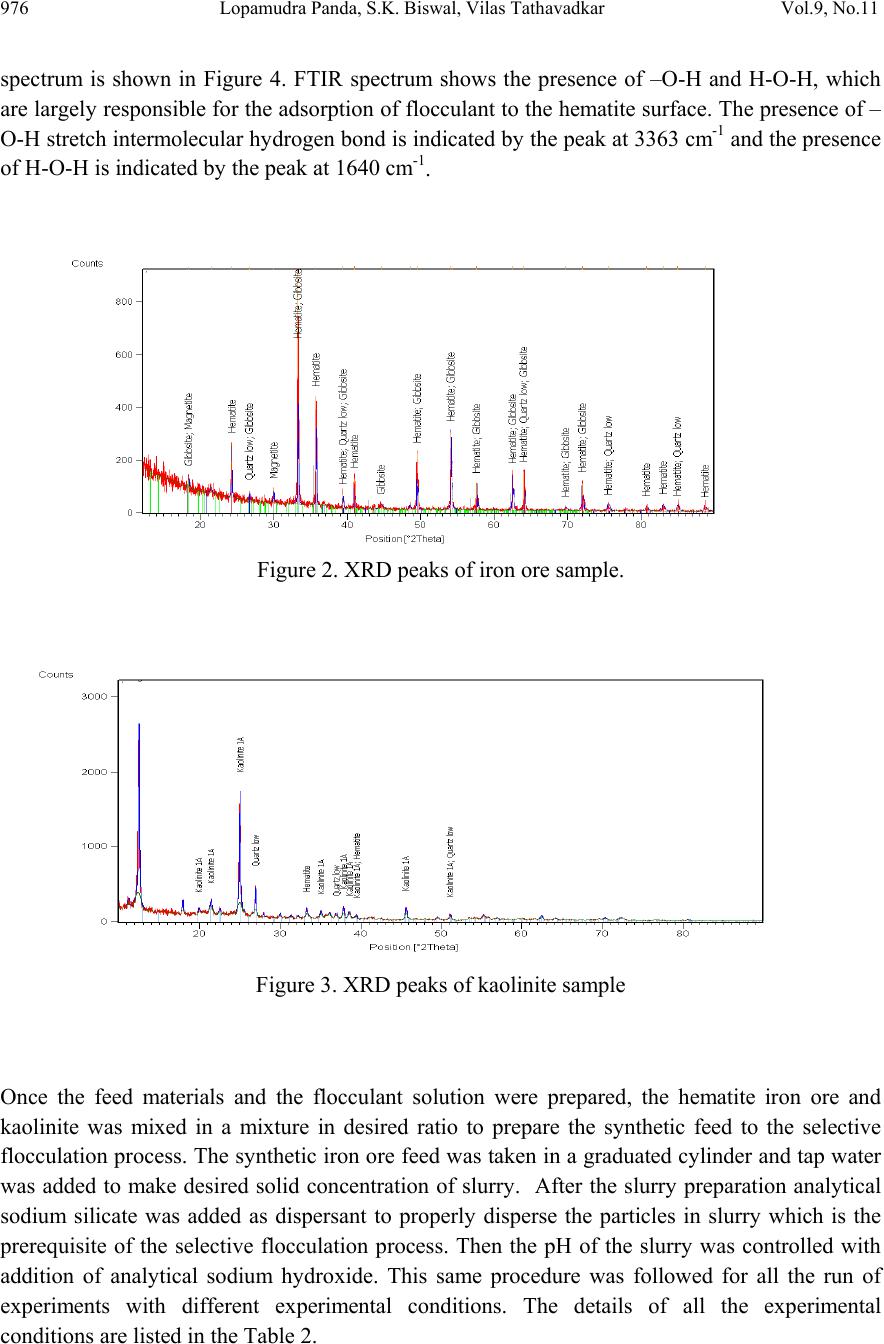 976 Lopamudra Panda , S.K. Biswal, V i l a s T athavadkar Vol.9, No.11 spectrum is shown in Figure 4. FTIR spectrum shows the presence of –O-H and H-O-H, which are largely responsible for the adso rption of flocculant to the hematite surface. The presence of – O-H stretch intermolecular hydrogen bond is indicated by the peak at 3363 cm-1 and the presence of H-O-H is indicated by the peak at 1640 cm-1. Figure 2. XRD peaks of iron ore sample. Figure 3. XRD peaks of kaolinite sample Once the feed materials and the flocculant solution were prepared, the hematite iron ore and kaolinite was mixed in a mixture in desired ratio to prepare the synthetic feed to the selective flocculation process. The synthetic iron or e feed was taken in a graduated cylinder and tap water was added to make desired solid concentration of slurry. After the slurry preparation analytical sodium silicate was added as dispersant to properly disperse the particles in slurry which is the prerequisite of the selective flocculation process. Then the pH of the slurry was controlled with addition of analytical sodium hydroxide. This same procedure was followed for all the run of experiments with different experimental conditions. The details of all the experimental conditions are listed in the Table 2. 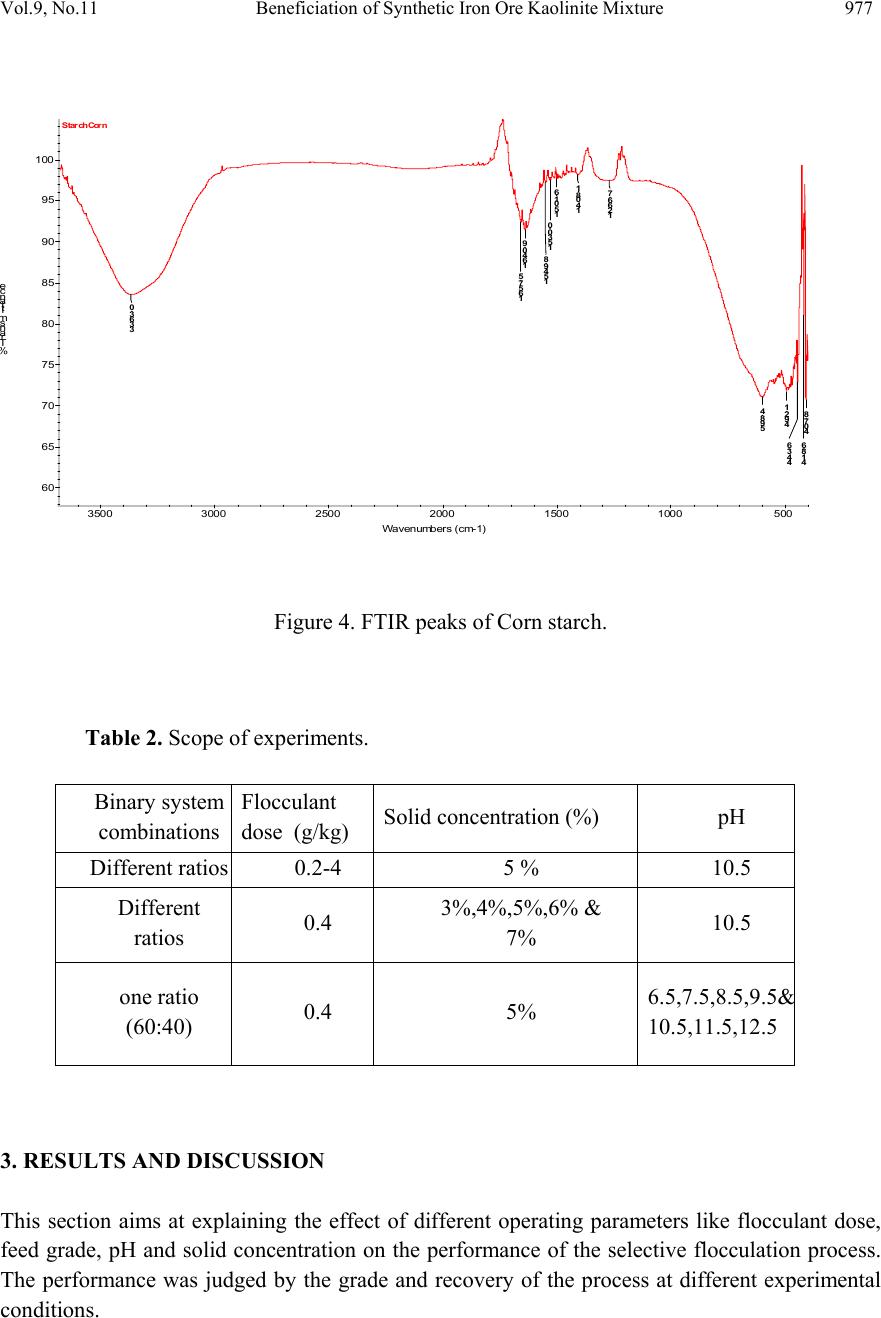 Vol.9, No.11 Beneficiation of Synthetic Iron Ore Kaolinite Mixture 977 4 0 7 . 8 4 1 8 . 6 4 4 3 . 6 4 9 2 . 1 5 9 8 . 4 1 2 6 6 . 7 1 4 0 8 . 1 1 5 0 1 . 6 1 5 3 0 . 0 1 5 4 9 . 8 1 6 4 0 . 9 1 6 5 7 . 5 3 3 6 3 . 0 Sta rch C orn 60 65 70 75 80 85 90 95 100 % T r a n s m i t t a n c e 500 1000 1500 20 00 2500 3000 3500 Wavenumbers (cm-1) Figure 4. FTIR peaks of Corn starch. Table 2. Scope of experiments. Binary system com binations Flocculant dose (g/kg) Solid concentration (%) pH Different ratios 0.2-4 5 % 10.5 Different ratios 0.4 3%,4%,5%,6% & 7% 10.5 one ratio (60:40) 0.4 5% 6.5,7.5,8.5,9.5 & 10.5,11.5,12.5 3. RESULTS AND DISCUSSION This section aims at explaining the effect of different operating parameters like flocculant dose, feed grade, pH and solid concentration on the performance of the selective flocculation process. The performance was judged by the grade and recovery of the process at different experimental conditions. 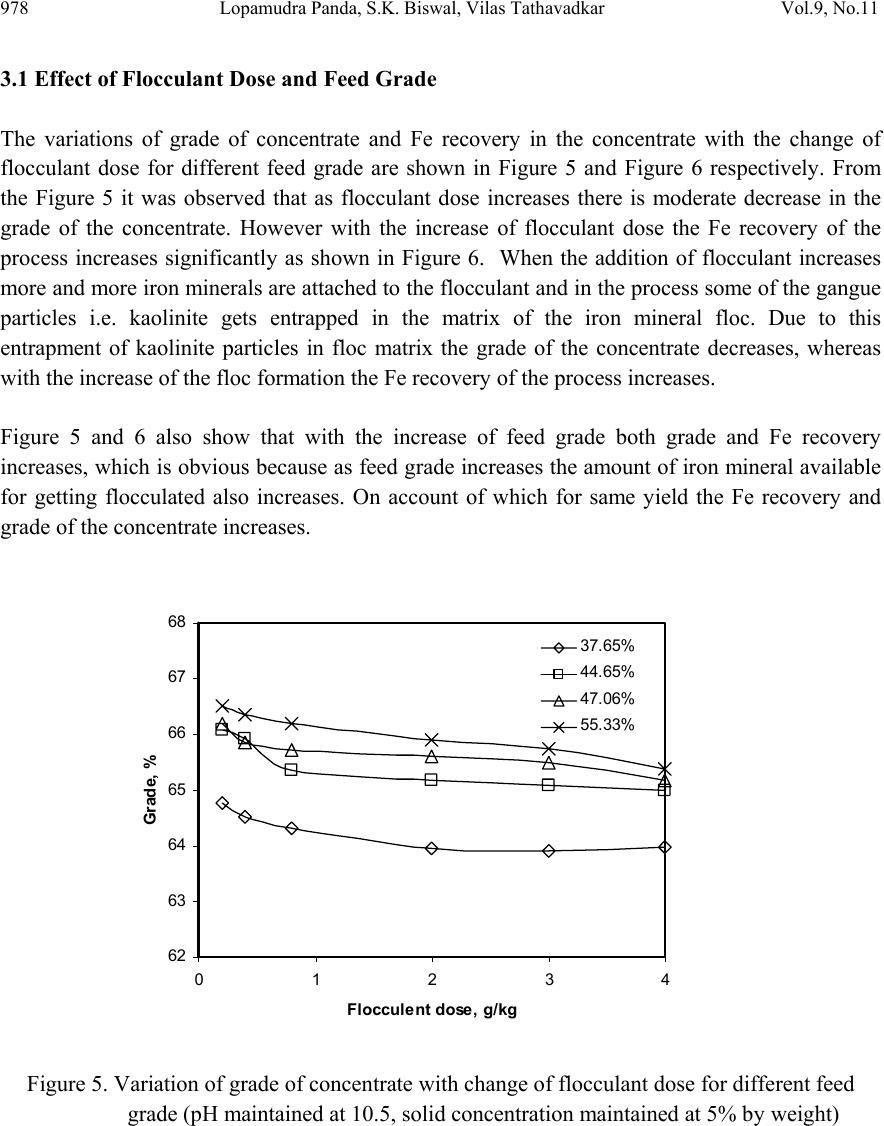 978 Lopamudra Panda , S.K. Biswal, V i l a s T athavadkar Vol.9, No.11 62 63 64 65 66 67 68 01234 Fl occulent dose , g/ kg Grade, % 37.65% 44.65% 47.06% 55.33% 3.1 Effect of Flocculant Dose and Feed Grade The variations of grade of concentrate and Fe recovery in the concentrate with the change of flocculant dose for different feed grade are shown in Figure 5 and Figure 6 respectively. From the Figure 5 it was observed that as flocculant dose increases there is moderate decrease in the grade of the concentrate. However with the increase of flocculant dose the Fe recovery of the process increases significantly as shown in Figure 6. When the addition of flocculant increases more and more iron minerals are attached to the flocculant and in the process some of the gangue particles i.e. kaolinite gets entrapped in the matrix of the iron mineral floc. Due to this entrapment of kaolinite particles in floc matrix the grade of the concentrate decreases, whereas with the increase of the floc formation the Fe recovery of the process increases. Figure 5 and 6 also show that with the increase of feed grade both grade and Fe recovery increases, which is obvious because as feed grade increases the amount of iron mineral available for getting flocculated also increases. On account of which for same yield the Fe recovery and grade of the concentrate increases. Figure 5. Variation of grade of concentrate with change of flocculant dose for different feed grade (pH maintained at 10.5, solid concentration maintained at 5% by weight) 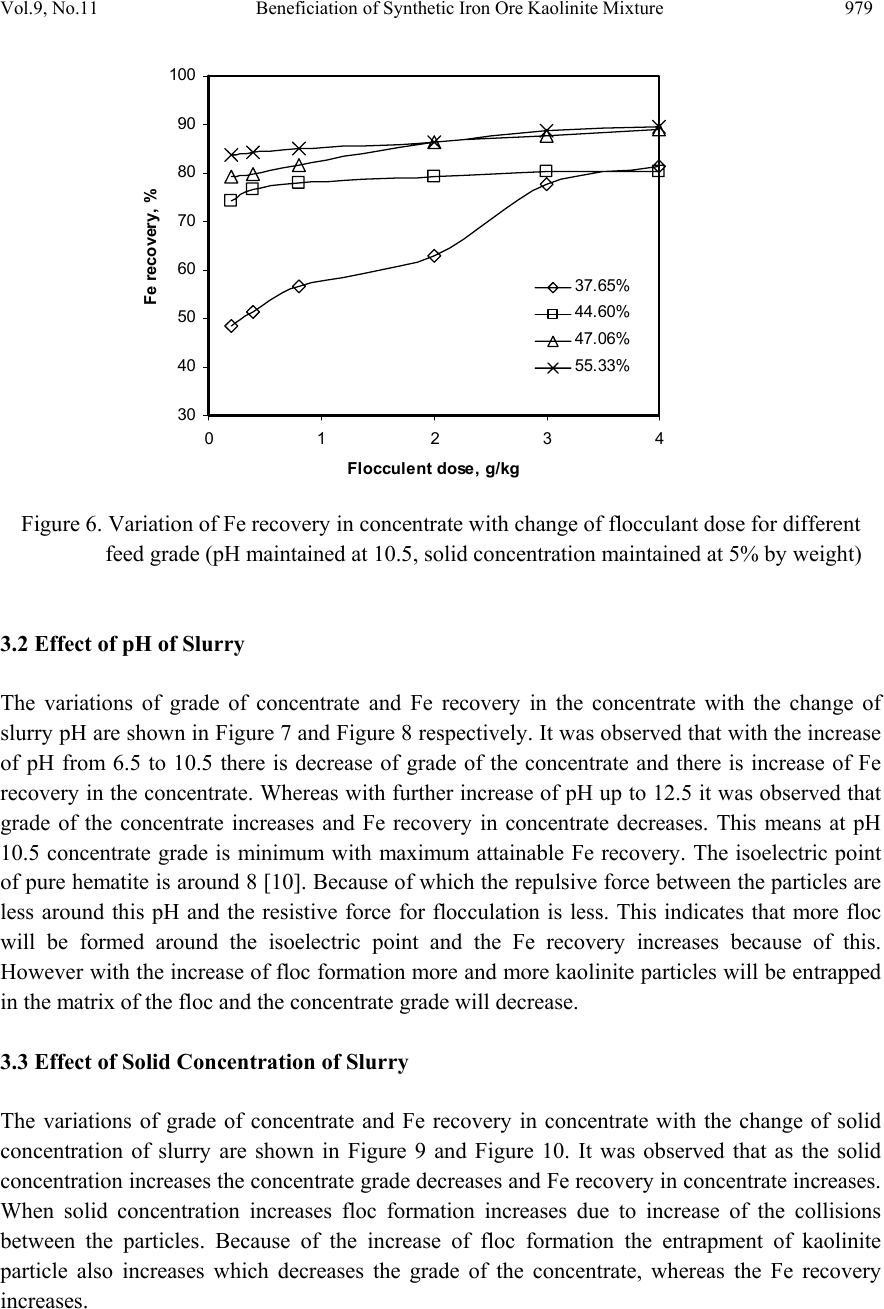 Vol.9, No.11 Beneficiation of Synthetic Iron Ore Kaolinite Mixture 979 30 40 50 60 70 80 90 100 01234 Flocculent dose , g / kg Fe recovery, % 37.65% 44.60% 47.06% 55.33% Figure 6. Variation of Fe recovery in concentrate with change of flocculant dose for different feed grade (pH maintained at 10.5, solid concentration maintained at 5% by weight) 3.2 Effect of pH of Slurry The variations of grade of concentrate and Fe recovery in the concentrate with the change of slurry pH are shown in Figure 7 and Figure 8 respectively. It w as observed that with the increase of pH from 6.5 to 10.5 there is decrease of grade of the concentrate and there is increase of Fe recovery in the concentrate. Whereas with further increase of pH up to 12.5 it was observed that grade of the concentrate increases and Fe recovery in concentrate decreases. This means at pH 10.5 concentrate grade is minimum with maximum attainable Fe recovery. The isoelectric point of pure hematite is around 8 [10]. Because of which the repulsive force between the particles are less around this pH and the resistive force for flocculation is less. This indicates that more floc will be formed around the isoelectric point and the Fe recovery increases because of this. However with the increase of floc formation more and more kaolinite particles will be entrapped in the matrix of the floc and the concentrate grade will decrease. 3.3 Effect of Solid Concentration of Slurry The variations of grade of concentrate and Fe recovery in concentrate with the change of solid concentration of slurry are shown in Figure 9 and Figure 10. It was observed that as the solid concentration increases the concentrate grade decreases and Fe recovery in concentrate increases. When solid concentration increases floc formation increases due to increase of the collisions between the particles. Because of the increase of floc formation the entrapment of kaolinite particle also increases which decreases the grade of the concentrate, whereas the Fe recovery increases. 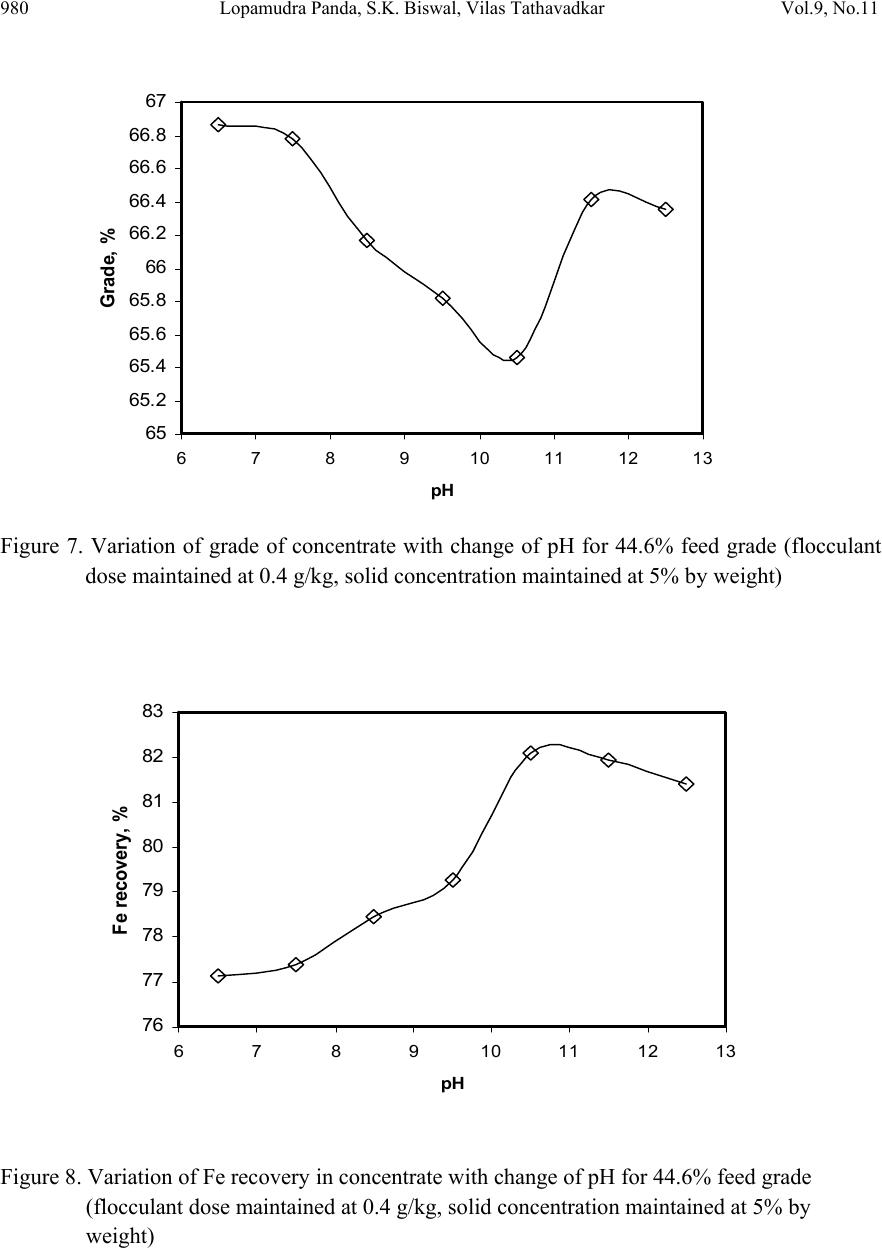 980 Lopamudra Panda , S.K. Biswal, V i l a s T athavadkar Vol.9, No.11 76 77 78 79 80 81 82 83 678910 1112 13 pH Fe r eco ver y, % 65 65. 2 65. 4 65. 6 65. 8 66 66. 2 66. 4 66. 6 66. 8 67 6 7 8 910111213 pH Grade, % Figure 7. Variation of grade of concentrate with change of pH for 44.6% feed grade (flocculant dose maintained at 0.4 g/kg, solid concentration maintained at 5% by weight) Figure 8. Variation of Fe recovery in concentrate with change of pH for 44.6% feed grade (flocculant dose maintained at 0.4 g/kg, solid concentration maintained at 5% by weight)  Vol.9, No.11 Beneficiation of Synthetic Iron Ore Kaolinite Mixture 981 63 64 65 66 67 2345678 Solid concentration, w t.% Gr ade, % 74 76 78 80 82 84 86 88 90 92 2345678 solid concentra tion, wt.% Fe r eco very, % Figure 9. Variation of grade of concentrate with change of solid concentration for 44.6% feed grade (flocculant dose maintained at 0.4g/kg, pH maintained at 10.5) Figure 10. Variation of Fe recovery in concentrate with change of solid concentration for 44.6% feed grade (flocculant dose maintained at 0.4g/ kg, pH maintained at 10.5)  982 Lopamudra Panda , S.K. Biswal, V i l a s T athavadkar Vol.9, No.11 4. CONCLUSION The use of low grade iron ore will increase day by day to meet the high demand of steel. Because of which beneficiation activities of these low grade iron ore will also increase and more and more tailings will be generated in the process. To beneficiate these tailings selective flocculation is going to be an alternative process. The success of the selective flocculation depends on the proper selection and preparation of flocculent. It was observed from this work that the grade and recovery of the process depends strongly on the flocculant dose, pH and solid concentration of the slurry. It was also observed that there exists an optimum pH near to the isoelctric point of hematite where there is maximum Fe recovery. ACKNOWLEDGEMENT The authors would like to gracefully acknowledge the funding given by CSIR, India through project NWP – 31 for carrying out this work. The authors would also like to thank Prof. B.K. Mishra, Director, IMMT, Bhubaneswar and management, Tata Steel Ltd., Jamshedpur for giving permission to publish this paper. REFERENCES [1] Attia, Y.A., 1977, “Development of a selective flocculation process for a complex copper ore”. International Journal of Mineral Processing, vol.4, pp.209-225. [2] Pradip and Moudgil,B.M.,1991,“Selective flocculation of tribasic calcium Phosphate from mixtures with quartz using polyacrylic acid flocculant”. International Journal of Mineral Processing, vol.32, pp. 271-281. [3] Sresty, G.C. and Somasundaran, P, 1980,“Selective Flocculation of Synthetic Mineral Mixtures using modified polymers”. International Journal of Mineral Processing, vol.6, pp. 303-320. [4] Friend, J. P. and Kitchener, J. A., 1973, “ Some physico-chemical aspects of the separation minerals by selective flocculation of finely-divided minerals by selective flocculation”, Chemical Engineering Science, vol-28, pp. 1071- 1080. [5] Mandre, N.R. and Panigrahi, D., 1997, “Studies on selective flocculation of complex sulphides using cellulose xanthate”. International Journal of Mineral Processing.,vol-50, pp- 177-186. [6] Weisseborn, P.K., Warren, L.J., Dunn, J.G., 1994, “Selective flocculation of ultra fine iron ore.1.Mechanisim of adsorption of starch into hematite”.Colloids and Surfaces, voL-99, pp 11-27. [7] Weisseborn, P.K., 1996, “Behavior of amylopectin and amylose components of starch in the selective flocculation of ultrafine iron ore”. International Journal of Mineral Processing , 47, pp 197-211. [8] Rao, K. Hanumantha. and Narasimhan, K.S., 1984, “Selective Flocculation Applied to Barsuan Iron Ore Tailings”. International Journal of Mineral Processing, vol - 14 pp. 67- 75.  Vol.9, No.11 Beneficiation of Synthetic Iron Ore Kaolinite Mixture 983 [9] Rao,K. Hanumantha, Nayak, K.R., Mahapatra, S.N., Narasimhan, K.S.,1985,“Selective flocculation for the recovery of iron in Kudremukh tailings”. Mining Engineering., vol - 37, pp. 1312-1315. [10] Tejeda Ramos., M.M., Ontiveros, A., Viota, J.L and Duran, J.D.G., J., 2003, “Interfacial and rheological properties of humic acid/hema tite suspensions”. Colloid Interface Science, 268, pp. 85–95. |

