Agricultural Sciences
Vol.4 No.6A(2013), Article ID:33717,10 pages DOI:10.4236/as.2013.46A002
Population structure and reproductive parameters of the Longneck croaker, Pseudotolithus typus (Pisces, Bleeker, 1863) in nearshore waters of Benin (West Africa) and their implications for management
![]()
1Département de Zoologie, Université d’Abomey-Calavi, Cotonou, Benin; *Corresponding Author: esossoukpe@yahoo.fr
2Department of Marine and Fisheries Sciences, University of Ghana, Legon, Ghana
3Water Research Institute, Council for Scientific and Industrial Research (CSIR), Accra, Ghana
Copyright © 2013 Edmond Sossoukpe et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 15 March 2013; revised 20 April 2013; accepted 15 May 2013
Keywords: Pseudotolithus typus; Benin Nearshore Waters; Sex Ratio; Size at First Capture; Size at First Sexual Maturation; Fisheries Management
ABSTRACT
Pseudotolithus typus is one of the two commercially important Sciaenids off Benin nearshore waters mainly fished by beach seining. Unfortunately, since 1994, the production of this species has been decreasing, and increasingly more small-sized fishes are regularly harvested, while little is known about the species’ population structure and its life history. Therefore, population structure, probability of capture and size at first capture were investigated using length-frequency data of 1144 specimen sampled from beach seine hauls over a period of 18 months. A total of 54.3% of this population was immature, confirming the domination of smallsized fishes in the catches. Gonad maturation stages were also examined. Frequency distribution of oocyte size exhibited two cohorts of mature oocytes suggesting two spawning periods per year. Monthly averages of gonado-somatic index confirmed that P. typus spawned twice a year during the major warm season (March - May) and during the transition minor warm to minor cold season in October - November. Length at first capture (L75 = 22.76 cm) was smaller than the length at first sexual maturation (L50% = 23.6 cm) indicating a heavy pressure of the beach seine on this resource. To give each fish the chance of reproducing at least once in its lifetime to recruit into the stock, necessary measures such as the size-limit regulation by gradually increasing beach seine mesh size should be developed. Community-based management of the nearshore fishery could contribute to reducing fishing effort during the reproductive periods from February to May and from October to December each year.
1. INTRODUCTION
Many of the world’s fish populations are overexploited, and probably to meet the fish consumption requirements of the human population (Milton et al., 2002 [1]; Nunoo et al., 2006 [2]; Fazli et al., 2007 [3] and Narges et al., 2011 [4]). Apart from being a cheap source of highly nutritive protein, fish contains essential nutrients required by the body (Sikoki et al., 1999 [5]).
The fisheries sub-sector is important to Benin on many fronts. For example, as a source of foreign income, the sub-sector contributed US $12 million to Benin’s total receipts of agricultural non traditional export products in 2008 (INSAE, 2009 [6]). Fish is also a preferred source of animal protein in the Beninese diet (approximately 55%), with a consumption of approximately 14.4 kg per person per annum (Direction des Pêches, 2011 [7]). The fish requirement for the population (8 millions, INSAE, 2003 [8]) was estimated to be approximately 96,000 metric tons. The fishery sector supports an informal workforce of 1.5 million people (approximately 18% of the population), which includes fishermen, fish processors and traders, most of whom are women (Anato, 1999 [9]).
The dependence of the Beninese on fish has resulted in a demand that far outstrips the total local production from capture fisheries (both marine and inland waters). In 2011, for example, the total production was 37,784 metric tons, while the demand was approximately 120,000 metric tons (Direction des Pêches, 2011 [7]). In 2005, the total value of fish imported into Benin was US $80 million. Approximately 33% of the local fish production is from marine waters. The marine fisheries sector in Benin is dominated by artisanal fisheries, which contribute approximately three-quarters of the total marine production (Gbaguidi, 2000 [10]). The species of high market value caught by artisanal fisheries are Sciaenids (21.75%), Polynemids (08.77%), Lutjanids (05.52%) and Sparids (04.07%) (Anato, 1996 [11]). Sciaenids constitute a large and varied family of fishes that are closely related to snappers, but differ in that the spinous dorsal fin is short, and the adipose tissue is much longer than the anal fin, which has only one or two spines (Edwards et al., 2001 [12]). This family comprises croakers, drums, meagres and weakfishes. Approximately 70 genera and 270 species are known, with 14 species occurring along the Gulf of Guinea on the coast of West Africa (Edwards et al., 2001 [12]).
The genus Pseudotolithus (Family: Sciaenidae) (croakers) constitutes an abundant and commercially-important fish group in Benin’s nearshore waters (Gbaguidi, 2000 [10], 2001 [13], Sossoukpe, 2011 [14]) and throughout the Atlantic coast of West Africa (Bayagbona, 1969 [15]). Pseudotolithus senegalensis and P. typus are widely distributed along the coast of tropical West Africa from Senegal to Angola (Edwards, et al., 2001 [12]). These two Sciaenids are easily mistaken. Numbers of spines at dorsal fin and Inter-Orbital length were the two discriminative morpho-meristic factors that could be used to reinforce macroscopically the difference between P. senegalensis and P. typus even at post juvenile stage (Sossoukpe, 2011 [14]).
Unfortunately, since 1994, the production of Pseudotolithus has been decreasing, and increasingly more small-sized fishes are regularly harvested (Gbaguidi, 1999 [16], 2000 [10], Sossoukpe, 2013 [17]). Larger specimens do not exceed 80 cm total length (TL) (Gbaguidi, 1999 [16], 2000 [10]). While P. senegalensis is widely studied (Longhurt, 1966 [18]; Troadec, 1971 [19]; N’Jock, 1990 [20]; Domain et al., 2000 [21]; Sidibé, 2003 [22]), there is a dearth of information on the reproduction of P. typus. However, information on sex ratio, size at first sexual maturation, spawning seasons and fecundity could be a useful input in deciding on sustainable management measures.
For example, the fluctuations of the sex ratio according to fish size provide a useful tool to examine the biological characteristics of the fish species, such as sexual inversion, longevity in relation to sex, vulnerability to fishing gear and the spatial, seasonal and even daily distribution of species (Aboussouan and Lahaye, 1979 [23]). Fecundity regulates the numerical abundance of the fish population and affects fish stocks.
The current study presents this information for Pseudotolithus typus to contribute to management and conservation policies for further development of this fishery in Benin.
2. MATERIALS AND METHODS
2.1. Study Area, Fish Sampling and Data Collection
Coastal waters serve as an important spawning area as well as a nursery zone for juveniles (Blaber et al., 1995 [24]). For this reason, the study of population structure and reproduction of P. typus was performed in the nearshore waters of Benin (West Africa) (Figure 1). Samples were taken weekly from commercial beach seine hauls for 18 months (March 2008 to August 2009) along Benin coast from Djako at Cotonou city (06˚20'51"N, 2˚21'58"E) to the village of Djègbadji at Ouidah city (06˚20'36"N, 02˚14'56"E). A total number of 1395 fish specimens were sampled from the catches and 1144 specimens were sexed. The samples were conveyed in thermos cool boxes to the laboratory.
Specimens were measured [total length (TL) and standard length (SL)] to the nearest 1.0 millimetre with a fish measuring board then weighed to the nearest 0.1 gram with an electronic balance. Pressure was applied to the ventral abdominal wall to determine if gonads were fully matured (indicated by expulsion of oocytes). Specimens were dissected and gonads were removed for weight measurements. Sex was determined by macroscopic examination of gonads. Gonad maturation stages were estimated using a scale of gonad maturation stages described by King (1995 [25]). Female stages were: 1) resting (juvenile)—ovary undeveloped, small and translucent with oocytes invisible to naked eye; 2) developing—ovary opaque, orange colour with oocytes opaque and visible to naked eye; 3) ripe—fully developed ovary fills ventral region of the abdominal cavity with translucent, large and round postvitellogenic eggs; 4) spawning—ovary releases eggs when pressed with large, translucent oocytes; some are free in the ovary (diameter 0.65 - 0.97 mm); 5) spent (postspawning)—shrinking or slack ovary with some residual eggs. Male maturation stages were: 1) immature (juveniles)—undeveloped testis consisting of a translucent filament; 2) early maturation— intermediate size testis having a very light yellow or tan colour; 3) advanced maturation—large testis, opaque white or light tan colour; 4) ripe testis—fully developed testis, pressure applied to ventrum expulses white milt; and 5) post spawning—flaccid testis without milt.
 (a)
(a) (b)
(b)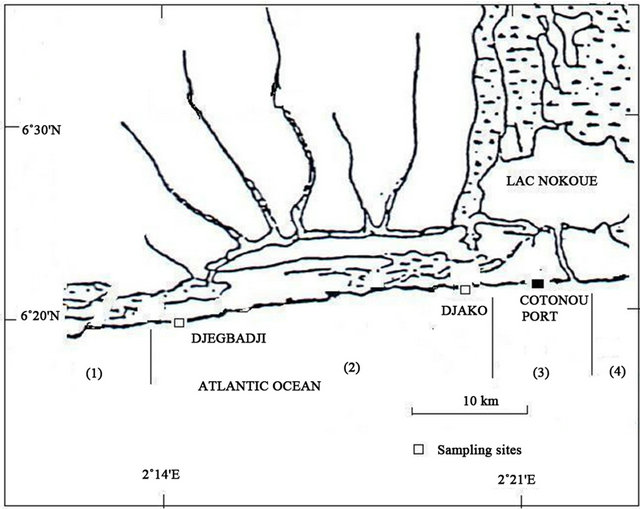 (c)
(c)
Figure 1. Maps showing Benin in Africa (a) of Benin (b) and sampling sites (c).
Subsequent to staging, gonads were fixed in 5% formalin. After 7 days, preserved gonads were removed from formalin and stored in 75% ethanol to avoid shrinking of oocytes with reduction of egg diameter. Fecundity was determined from 25 late-ripening ovaries (stage III) collected at each site during February to March for P. typus (Figure 2). Batch fecundity was estimated as a total number of mature oocytes extrapolated from three 0.1 g samples taken from each ovary (samples from anterior, middle and posterior portions of the ovary). After at least two weeks the specimen tubes were shaken vigorously to liberate the eggs from the ovarian tissue. Pooled oocytes from all three areas were counted in a Petri dish under a binocular microscope (10 × 1.6). Oocytes having yellow-orange colour and range 0.45 - 0.97 mm in diameter were considered large (mature) and then counted. Diameters of 10 mature oocytes from each of 25 gonads were measured using an ocular micrometer attached to a dissecting stereomicroscope. The diameters of ova from representative ovaries of the five maturity stages were also measured.
Some organs such as liver could provide required energy for appropriate functioning of the fish body. For this reason, liver of each individual fish was weighed (precision 0.001 g).
2.2. Data Analysis
The size frequency distribution was analyzed using 2 cm class interval (standard length) to plot a histogram to determine the type of distribution, which characterizes the fish population.
Sex ratio was calculated for different months and size groups of fish. The sex ratio was tested for equality for different months using Chi-square test. The Gonadosomatic Index (GSI) was used to determine the spawning period using the formula:
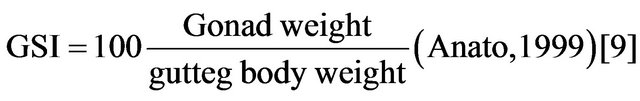
The reproduction or spawning period was determined by the changes in the monthly GSI. The period between the beginning of the increase of the GSI and its decrease corresponds to the reproduction period. The egg-laying time corresponds to the maximum value of the GSI.
Fishes belonging to maturity stage II and above were considered as matured fish and used for calculating the size at first sexual maturation (L50). The L50 was estimated as the size at which 50% of individuals were classified as “mature” or “advanced maturation” stage (Albaret, 1977 [26]). The L50 was estimated from a sigmoid curve constructed with the percentage of mature individuals and associated size class categories. A frequency distribution was plotted for oocytes diameter based on ovaries in the top 10th percentile of GSI values.
Figure 2. Removed internal organs showing republic ovary at maturation stage III.
Fecundity was estimated for mature ovaries as:

where Wg is the gonad weight, “n” is mean number of oocytes in the sample ovary, “p” is mean weight of the sample ovary.
The linear relationship of fecundity with gonad weight was examined as: Log(F) = bLog(Wg) + Log(a), where F is the fecundity, Wg is the gonad weight, “a” is the intercept and “b” is the slope.
The probability of capture provides a clear indication of the estimated real size of fish in the fishing area that are being caught by specific gear. Probability of capture is also an important tool for fishery managers who, by regulating the minimum mesh size of a fishing fleet, can mostly determine what should be the minimum size of the target species of a fishery. The probability of capture was estimated by backward extrapolation of the descending limb of the length converted catch curve. A selectivity curve was generated using linear regression fitted to the ascending data points from the plot of probability of capture against length, which was used to estimate the final value of L25, L50 and L75 (i.e., lengths at which 25%, 50% and 75% of the fish will be vulnerable to the gear, respectively).
Estimates of length-at-first capture (L50) were derived from the probabilities of capture generated from the catch curve analysis output by FiSAT II (version 1.2.2., 2005).
3. RESULTS AND DISCUSSION
3.1. Population Structure and Sex Ratio
The population was distributed as indicated in Table 1. Only 45.7% of this population was mature. The distribution of population size structure was unimodal (Figure 3) with a modal class interval of 24.0 cm.
A total of 54.3% of individuals sampled were imamture (sexed and unsexed). Gbaguidi (1999) [15] reported that 72% of individuals of Pseudotolithus species caught by beach seine from Benin nearshore waters were also juveniles. Nunoo (2003) [27] reported that 90% of individuals of fish assemblages were juveniles in the beach seine hauls at Sakumono in Ghana throughout the year indicating the dependence on the nearshore as a nursery for juveniles. These finding agrees with that of Ashong (1999) [28] for Sakumono between 1998 and 1999. However, juvenile fishes formed about 53% of individuals in beach seine hauls at False Bay, South Africa (Clark et al., 1996 [29]).
The data on sex ratio is presented in Table 2. The ratio of males to females was found to be 0.79:1. Test of significance of difference revealed that in March, July, October and December 2008 and in February - March 2009 the number of males and females differ significantly (X2 = 10.72, P < 0.001; X2 = 11.58, P < 0.001; X2 = 8.49, P < 0.01; X2 = 6.46, P < 0.01; X2 = 10.32, P < 0.001; X2 =
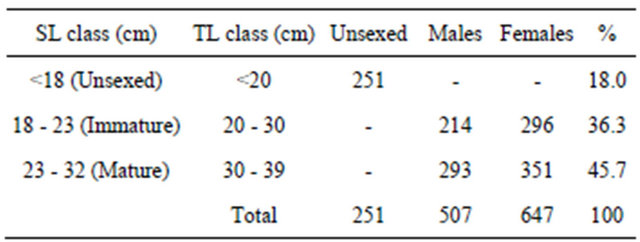
Table 1. Sampling distribution by gender of P. typus from Benin nearshore waters between March 2008 and August 2009.
12.42, P < 0.001 respectively). In the other months, the difference was insignificant. The Chi-square test of pooled data did not show any significant difference (X2 = 0.32, P < 0.05).
The sex-ratio (males to females) was widely variable from one month to another. During the reproduction period, the number of females outstrips those of males. For the pooled data, it was about 0.79:1. Caverivière (1982) [30] reported that, the dominance of females at the ad-
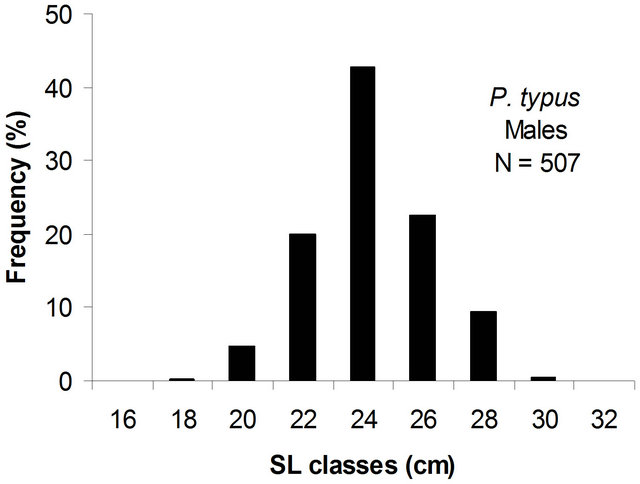
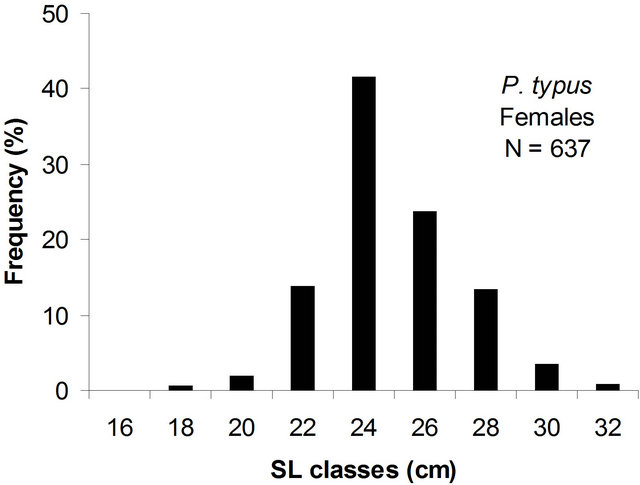
Figure 3. Size structure of males and females of P. typus sampled from Benin nearshore waters between March 2008 and August 2009.

Table 2. Monthly variations in sex ratio of P. typus from Benin nearshore waters between March 2008 and August 2009.
vanced age could be explained by: an availability or a high vulnerability of females; or a higher natural mortality of males; a sexual inversion; a differential growth or a mere differential sexual migration. According to Fontana (1979) [31], the sex ratio was dominated by females of P. typus beyond 42 cm (TL) while in Guinea the sex ratio did not exceed 0.5:1 only from length 54 cm to reach 1:1 at 68 cm (TL). Variations of the sex-ratio according to the size, which are observed, can have a considerable influence on the fertility of stocks depending on whether majority of the adult individuals captured were females or males. Finally, it is necessary to note that the sex-ratio of the population is calculated from catches and that the observed variations can be an indication of catchability between the two sexes. However, sex ratio is influenced by many factors. According to Bertin (1958) [32] and Lissia Frau (1966) [33], the difficulties to determine the sex of immature and hermaphrodite individuals could greatly affect the proportion of males and females obtained.
3.2. Development of Ova to Maturity
The size frequency distribution of ova in the stages I - V of maturity are shown in Figures 4-6. The frequency histograms of diameters of various maturity stages showed
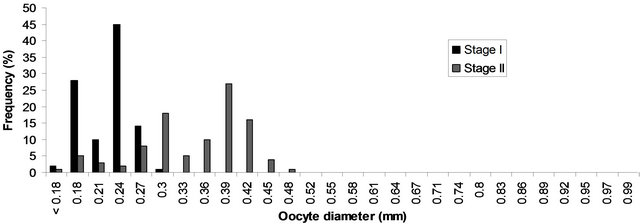
Figure 4. Size frequency distribution of oocytes in ovaries at maturation stage I and II of P. typus from Benin nearshore waters sampled between March 2008 and August 2009.

Figure 5. Size frequency distribution of oocytes in ovaries at maturation stage III and IV of P. typus from Benin nearshore waters sampled between March 2008 and August 2009.
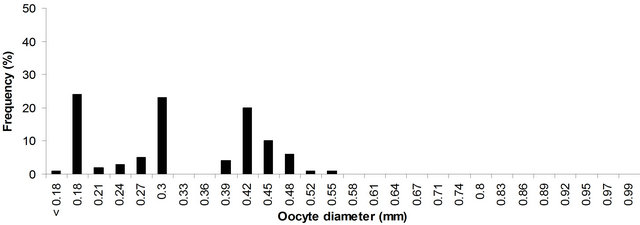
Figure 6. Size frequency distribution of oocytes in ovaries at maturation stage V of P. typus from Benin nearshore waters sampled between March 2008 and August 2009.
that in stage I (immature ovary), majority of the ova were 0.18 - 0.21 mm (representing the immature stock of ova), and a few ones measured up to 0.30 mm. The immature stock of ova was found in all the subsequent stages. In stage II (maturing ovary), a batch of ova separated from the general stock forming a mode at 0.18 - 0.21 mm, another distinct mode at 0.27 - 0.30 mm forming the first batch of maturing ova was also formed. In stage III, (mature ovary), the first batch of maturing ova which appeared in stage II, developed further and formed a mode at 0.0.30 - 0.0.33. In addition a new batch of maturing ova with mode at 0.24 - 0.27 mm contributed to its appearance. At this stage the mature group of ova was well demarcated from maturing and immature ones by mode a mode 0.74 - 0.87 mm. Stage IV (ripe ovary), contained all the four stages of ova. The mature group of ova with mode at 0.35 - 0.38 mm, which was found in stage III, developed further and reached a mode at 0.74 - 0.97 mm, these ova were ripe and were ready for spawning. In addition to this group, modes at 0.32 - 0.35 mm representing the mature ova, 0.17 - 0.20 mm and a fraction at 0.23 - 0.26 mm also took place. In stage V (spent ovary), the ripe ova that had been at 0.74 - 0.97 mm in stage IV were already extruded, and there were only immature, maturing and few residual of mature ova. Generally, the maturing and mature ova passed in succession to advanced maturity stages and fresh batches of maturing ova separated from the immature stock.
3.3. Spawning Frequency
As can be seen from Figures 4-6, in an advanced stage of ovary three distinct groups of maturing and mature ova at 0.21 - 0.32, 0.32 - 0.35 and 0.74 - 0.97 mm are separated from the immature ones (0.18 - 0.21). As the fish approaches the spawning season, the most advanced group of ova at 0.74 - 0.97 mm (83% of total oocytes in mature ovary, Figure 7) was separated distinctly and released from the follicular chamber. By the time the next group at 0.45 - 0.48 mm (10% of total oocytes in mature ovary, Figure 6) is approaching the ripe stage suggests a second spawning season.
Monthly averages for GSI (Figure 8) peaked twice a year, in February and in October, suggesting two spawning periods per year. The first one occurred from February to May (range 3.285 ± 0.03 - 1.801 ± 0.03) and the
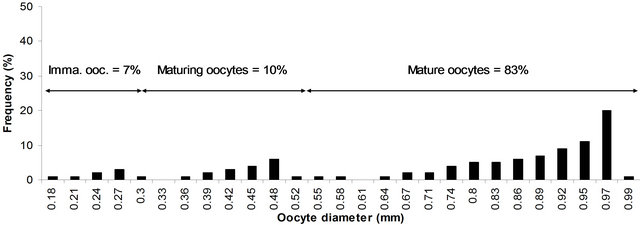
Figure 7. Size frequency distribution of oocytes in mature ovaries of P. typus from Benin nearshore waters sampled between March 2008 and August 2009.
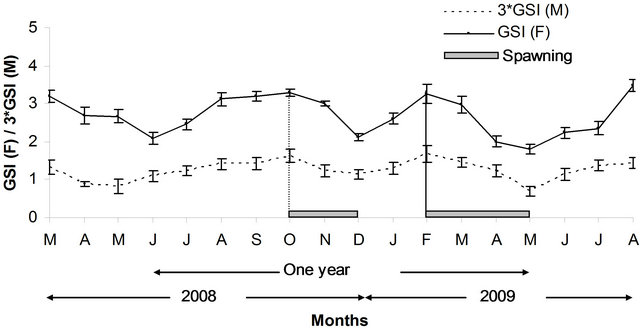
Figure 8. Variation of monthly averages of GSI of males and females of P. typus from Benin nearshore waters with indication of spawning seasons.
second, from October to December (range 3.259 ± 0.02 - 2.122 ± 0.04). Proportions of ripe males and females in each month were correlated (r2 = 0.65; P < 0.01). The percentage of individuals with mature gonads (stages III and V) and mean monthly GSI were correlated for both males (r2 = 0.38; P < 0.05) and females (r2 = 0.73; P < 0.01), providing confirmation that P. typus spawned twice a year. The first spawning occurs during the major hydrological warm season in Benin (February - May) and the second including the minor hydrological warm season (in November). These findings confirm those of Longhurst (1966) [18] who reported that P. typus in Nigeria spawn mainly during the warm season in waters where temperature is high or equal to 27.5˚C. In Congo, using percentages of mature females, Poinsard and Troadec (1966) [34] fixed the spawning during the minor and major warm seasons. Several observations by the different authors, and summed up well by Troadec (1971) [19], indicated that spawning would take place at shallow depths and probably at the neighborhood of stream mouths, rivers or lagoons.
3.4. Size at First Sexual Maturation
The proportion of mature individuals increased rapidly between 21 and 28 cm SL for both males and females (Figure 9). The size at which 50% of individuals were mature was 23.3 cm (SL, males) and 23.8 cm (SL, females). The smallest size at which mature specimens were found was 18.4 cm (Stage II, 155 g ungutted weight) and 19.5 cm (stage II, 185 g ungutted weight) in male and female, respectively. The total length at which 50% of P. typus individuals became mature (TL50) was 30.1 cm. This value was found to be close to what was reported in Cameroon (N’Jock, 1990 [20]) and different from what was reported in Guinea (Sidibé, 2003 [22]) which however shares the same Gulf of Guinea as Benin. In conclusion, the size at first sexual maturation is relatively variable with the species and its bio-geographical zone.
3.5. Probability and Length of First Capture
The length of first capture of 50% and 75% of the fish individuals was 22.76 and 23.36 cm, respectively (Figure 10). In the present investigation, the length at first capture of 75% of P. typus species (L75% = 23.36 cm) was smaller than the length of first sexual maturation (L50 = 23.8 cm, Figure 9). This situation, of harvesting prespawning fishes, may cause a greater reduction in the catch in near future. To maintain a population in equilibrium it is of great importance to give each fish the chance of reproducing at least once in its lifetime to replenish the stock, and therefore the length at first capture, Lc should be bigger than the length at first sexual maturation, Lm (Narges et al., 2011 [4]).
In addition, several studies on recruitment show that the number of recruits per year would depend on abundance as well as the strategy of reproduction of the stock and the variability of their environment (Blaxter, 1973 [35]; Cushing, 1975 [36]; Beyer and Laurence, 1980 [37]; Walters, 1986 [38], Sinclair, 1988 [39]; Cury, 1989) [40]. Conditions of the area could play a fundamental role in the regulation of recruitment by their influence on the survival of eggs, larvae and juvenile as well as on the fertility of the reproductive stock probably based on trophic relationships.
3.6. Fecundity
Overall, fecundity (measured as the number of mature oocytes in the ovary) was strongly correlated with gonad weight (r2 = 0.801; P < 0.05) (Figure 11). Body length was correlated with fecundity but the body weight revealed no general association with fecundity (Fa = 4.7666 LogSL − 1.8764, r2 = 0.3362, P < 0.05; Fa = 0.5782 LogWe + 3.4061, r2 = 0.0232, P > 0.05). The Lowest and highest fecundities recorded for individual fish were 32,282 oocytes (24.5 cm SL, 221.4 g We) and 129,740 oocytes (27.5 cm SL, 249.5 g We), respectively. The mean fecundity of P. typus of Benin nearshore waters was about 63,655 ± 1080 (P < 0.05).

Figure 9. Graphs showing the size at first sexual maturation for both genders of P. typus from Benin nearshore waters sampled between March 2008 and August 2009.
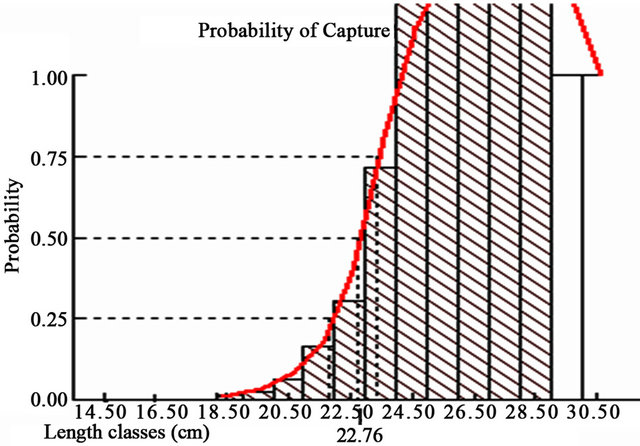
Figure 10. Probability and size at first capture of P. typus individuals (output from FISAT).

Figure 11. Best fit relationships between fecundity (F) and gonad weight (Wg).
4. CONCLUSION
In theory, equilibrium life history strategies should be associated with significant density-dependent recruitment, such that minimum or optimal densities of spawners could be estimated. Maintenance of a healthy population of spawners is essential for sustainable fisheries. Nonetheless, the high economic value of the Longneck croaker has induced many fishermen to adopt unsustainable fishing practices, such as beach seining to meet the most basic needs of their families. To improve chances for a sustainable Longneck croaker fishery in the nearshore waters of Benin, better enforcement of fishing regulations governing the open access coastal fishery coupled with community-based management of the nearshore fishery that reduced fishing effort during the reproductive periods from February to May and from October to December yearly will be beneficial.
5. ACKOWLEDGEMENTS
This work is part of a Ph.D. thesis undertaken at Department of Marine and Fisheries Sciences, University of Ghana, Legon, (Ghana). We owe much gratitude to Mr E. Lamptey of Department of Oceanography and Fisheries, University of Ghana for his useful advice. The study was funded by the Government of Benin through the Ministry of High Study and Scientific Research.
REFERENCES
- Milton, D., Mazid, M.A., Haldar, G.C., Rahman, M.A. and Nurul Amin, S.M. (2002) Population dynamics and stock assessment of Hilsa Shad, Tenuolosa ilisha in Bangladesh. Asian Fisheries Society, 15, 123-128.
- Nunoo, F.K.E., Eggleston, D.B. and Vanderpuye, C.J. (2006) Abundance, biomass and species composition of nearshore fish assemblage in Ghana, West Africa. African Journal of Marine Science, 28, 689-696. doi:10.2989/18142320609504217
- Fazli, H., Zhang, Cl., Hay, D.E., Lee, C.W., Janbaz, A.A. and Borani, M.S. (2007) Population dynamics and stock assessment of common Kilka (Clupeonella cultriventris caspia) in the Caspian Sea. Fisheries Science, 73, 285- 294. doi:10.1111/j.1444-2906.2007.01334.x
- Narges, A., Preeta, K., Jasem, M., Gholam-reza, E. and Yahid, Y. (2011) Stock assessment of silver pomfret Pampus argenteus (Euphrasen, 1788) in the Northern Persian Gulf. Turkish Journal of Fisheries and Aquatic Sciences, 11, 63-65. doi:10.4194/trjfas.2011.0109
- Sikoki, F.D. and Otobotekere, A.J.T. (1999) Fisheries. In: Alagoa, E.C., Ed., The Land People of Bayelsa State Central Niger Delta, Onyoma Research Publications, Port Harcourt, 301-319.
- INSAE (2009) Enquête-cadre sur les filières agricoles pourvoyeuses de devises. Ministère de la Prospective, du Développement et de l’Evaluation de l’Action Publique du Bénin, Cotonou.
- Direction des Pêches (2011) Rapport annuel d’activités. Ministère de l’Agriculture, de l’Elevage et de la Pêche, République du Bénin, 72.
- INSAE (2003) Recensement général de la population et de l’habitation en République du Bénin. Ministère de la Prospective, du Développement et de l’Evaluation de l’Action Publique du Bénin, Cotonou.
- Anato, C.B. (1999) Les sparidae des cotes béninoises: Milieu de vie, pêche, présentation des espèces et biologie de Dentex angolensis Poll et Maul, 1953. Thèse de Doctorat d’Etat es Sciences; Faculté des Sciences, Tunis, 277.
- Gbaguidi, A. (2000) Statistiques de la pêche maritime artisanale. Direction des Pêches, 40.
- Anato, C.B. (1996) Les ressources marines vivantes du Golfe de Guinée de la Guinée Conakry à l’Angola: Cas du Bénin. Atelier Sous Régional du Comité Océanographique International UNESCO, 125, Cotonou, 5-7 Juin 1996, 43.
- Edwards, A.J., Anthony, C.G. and Abohweyere, P.O. (2001) A revision of Irvine’s marine fishes of tropical West Africa. Darwin Initiative Report, 2, 157.
- Gbaguidi, A. (2001) Etude de l’impact écologique et socio- économique de la senne de plage sur les moyens d’existence des communautés de pêche au Bénin. Programme des Moyens d’Existence Durable dans la Pêche. Rapport d’Etude. Direction des Pêches, 53.
- Sossoukpe, E. (2011) Ecological studies on Pseudotolithus spp (Sciaenidae) in Benin (West Africa) nearshore waters: Implications for conservation and management. Ph.D. Thesis, University of Ghana, Legon, 219.
- Bayagbona, E.O. (1969) Age determination and the bertalanffy growth parameters of Pseudotolithus typus and Pseudotolithus senegalensis using the “burnt otolith technique”. In: Actes Symposium Océanographie et Ressources Halieutiques Atlantique Tropical, UNESCO, Abidjan, 27, 349-359.
- Gbaguidi, A. (1999) Statistiques de la pêche maritime artisanale. Direction des Pêches, Cotonou, Bénin, 52.
- Sossoukpe, E., Nunoo, F.K.E., Ofori-Danson, P.K., Fiogbe, E.D. and Dankwa, H.R. (2013) Growth and mortality parameters of P. senegalensis and P. typus (Sciaenidae) in nearshore waters of Benin (West Africa) and their implications for management and conservation. Fisheries Research, 137, 70-80. doi:10.1016/j.fishres.2012.08.020
- Longhurst, A.R. (1966) Synopsis of biological data on West African croakers (Pseudotolithus typus, P. senegalensis and P. elongatus). FAO Fisheries Synopsis, 35, 50.
- Troadec, J.P. (1971) Biologie et dynamique d’un Sciaenidae africain, Pseudotolithus typus. Document Scientifique Centre de Recherche Océanographique, Abidjan, 2, 1-125.
- N’Jock, J.C. (1990) Les ressources démersales côtières du Cameroun: Biologie et exploitation des principales espèces ichtyologiques. Thèse de Doctorat 3ème Cycle, Université Aix-Marseille 2, Cymbium, 3e sér France, 187.
- Domain, F., Chavance, P. and Bah, A. (2000) Description des fonds du plateau continental. In: Domain, F., Chavance, R. and Diallo, A., Eds., La Pêche Côtière en Guinée. Ressources et Exploitation, Editions IRD/CNRHB, Paris, 159-171.
- Sidibé, A. (2003) Les ressources halieutiques démersales côtières de la Guinée. Exploitation, biologie et dynamique des principales espèces de la communauté à Sciaenidés. Thèse de Doctorat, Ensar, Rennes, 320.
- Aboussouan, A. and Lahaye J. (1979) Les potentialités des populations ichtyologiques. Fécondité et Ichtyoplancton, 6, 29-46.
- Blaber, S.J.M., Brewer, D.T. and Salini, J.P. (1995) Fish communities and the nursery role of shallow inshore waters of a tropical bay in the Gulf of Carpentaria, Australia. Estuarine, Coastal and Shelf Science, 40, 177-193. doi:10.1016/S0272-7714(05)80004-6
- King, M. (1995) Fisheries biology, assessment and management. Fishing News Books, Oxford, 341.
- Albaret, J.J. (1977) La reproduction de l’Albacore (Thinnus albacores) dans le Golfe de Guinée. Cahiers ORSTOM, Séries Océanographiques, 15, 389-419.
- Nunoo, F.K.E. (2003) Biotic, abiotic and anthropogenic controls of nearshore marine fish assemblage caught in beach seines in Sakunono, Ghana, and their management implications. Ph.D. Thesis, University of Ghana, Legon, 155.
- Ashong, M. (1999) The beach seine fishery of Sakumono. Bsc Dissertation, University of Ghana, Legon, 33.
- Clark, B.M., Bennett, B.A. and Lamberth, S.J. (1996) Factors affecting spatial variability in seine net catches of fish in the surf zone of False Bay, South Africa. Marine Ecology Progress Series, 131, 17-34. doi:10.3354/meps131017
- Caverivière, A. (1982) Les espèces démersales du plateau continental ivoirien. Biologie et exploitation. Thèse de Doctorat d’Etat Université, Aix-Marseille, 415.
- Fontana, A. (1979) Etude du stock démersal côtier congolais. Biologie des principales espèces exploitées. Propositions d’aménagement de la pêcherie. Thèse Doctorat Etat, Université Paris VII, Paris, 300.
- Bertin, L. (1958) Sexualité et fécondation. In: Grassé, Ed., Traite de Zoologie, Masson et Cie, Paris, 1584-1652.
- Lissia Frau, A.M. (1966) Ricerche sul differenziamento sessuale di Boops boops (L.) Bollet di Pesca Pisci Idrobiologia, XXI, Fascicul, 1, 9-22.
- Poinsard, F. and Troadec, J.P. (1966) Détermination de l’âge par la lecture des otolithes chez deux espèces de Sciaenidés Ouest-africains (Pseudotolithus senegalensis (C.V.) et P. typus (BLKR). Journal Constitutif Permanent International Exploration Mer, 291-307.
- Blaxter, J.H.S. (1973) The early life of history of fish. Springer Verlag, Berlin, 235.
- Cushing, D.H. (1975) Marine ecology and fisheries. Cambridge University Press, Cambridge, 200.
- Beyer, J.E. and Laurence, G.C. (1980) A stochastic model of larval fish growth. Ecological Modelling, 8, 109-132. doi:10.1016/0304-3800(80)90032-0
- Walters, C.J. (1986) Adaptative management of renewable resources. Macmillan Publishers, London, 375.
- Sinclair, P. (1988) Marine populations: An essay on population regulation and speciation. Washington Sea Grant Program, Seattle, 553-564.
- Cury, P. (1989) Approche modélisatrice des relations à court, moyen et long terme entre la dynamique des stocks de poissons pélagiques côtiers et les fluctuations climatiques. Thèse Doctorat, Université. Paris VII.

