American Journal of Analytical Chemistry
Vol.05 No.16(2014), Article ID:51704,3 pages
10.4236/ajac.2014.516113
On the Question of defining the association Constants by the Method of fluorescence quenching
Nikolay L. Lavrik, Nikolay M. Bazhin
Voevodsky Institute of Chemical Kinetics and Combustion SB RAS, Novosibirsk, Russian Federation
Email: lavrik@kinetics.nsc.ru
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 5 September 2014; revised 20 October 2014; accepted 5 November 2014
ABSTRACT
A study is made on the previously ignored problem of the dependence of a static fluorescence quenching Stern-Volmer constant 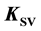 on the initial concentration of
on the initial concentration of 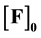 fluorophore F. This correlation is shown to exist. It is concluded that the Stern-Volmer quenching constant may be used as association constant K only with
fluorophore F. This correlation is shown to exist. It is concluded that the Stern-Volmer quenching constant may be used as association constant K only with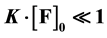 .
.
Keywords:
fluorescence quenching, associationconstants, Stern-Volmer Constant

1. Introduction
Determination of the association constants K is one of the most common tasks of physical chemistry, biochemistry, chemistry, etc. The constant K in equation (1) is taken as the association constant
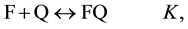 (1)
(1)
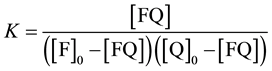 . (2)
. (2)
In (1) and (2), F and Q are the complexing reagents; FQ is the complex of reagents; 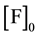 and
and 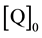 are the
are the
initial concentrations of F and Q according to preparation.
From equation (2) it easily follows [1] that the relative concentration (y) of complex FQ can be calculated from the equation
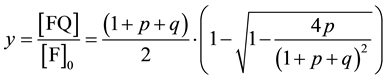 (3)
(3)
where
 . (4)
. (4)
One of the numerous methods to determine the value of K is the fluorescence one [2] . The basis of the described method is the assumption that the complexes FQ do not fluoresce (static quenching) and that the dynamic quenching of excited molecules F is absent.
The essence of this approach is that the fluorescence quenching constant 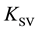 is estimated from the Stern-Volmer equation
is estimated from the Stern-Volmer equation
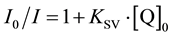 . (5)
. (5)
In (3), I0 and I are the fluorescence intensities of the fluorophore F in the absence and in the presence of quencher Q. The resulting constant  is taken as the K value [2]
is taken as the K value [2]
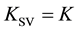 . (6)
. (6)
However, in the case of static quenching, the constant 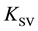 depends on the initial concentration of the fluorophore. This factor does not take into account usually [2] . The cause of such dependence is the formation of complexes FQ, which leads to a decrease in the concentration of free molecules F in contrast to the usual practice of using Stern-Volmer equation in the systems in which the concentration of the fluorophore does not depend on the concentration of the quencher.
depends on the initial concentration of the fluorophore. This factor does not take into account usually [2] . The cause of such dependence is the formation of complexes FQ, which leads to a decrease in the concentration of free molecules F in contrast to the usual practice of using Stern-Volmer equation in the systems in which the concentration of the fluorophore does not depend on the concentration of the quencher.
The present work is devoted to the extent of correction to the experimental Stern-Volmer constant 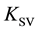 for the case of static fluorescence quenching under continuous illumination.
for the case of static fluorescence quenching under continuous illumination.
2. Fluorescence Quenching under the Conditions of nonfluorescent complex Formation
Consider now luminescence quenching in the presence of complex formation under stationary excitation. The following scheme holds for this case:
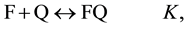



In (7)-(9), 

Thus, in general, from (10) the fluorescence intensity I is of the form

However, for a particular case of

In fluorescence quenching the 

Taking into account that

we get either

or

For the small values of р, we have

To determine the Stern-Volmer constants, 

on


Comparing (18) with (6) indicates that in the case of complex formation, the Stern-Volmer quenching constant 

Thus, 
constant. This correction to unity in the denominator of Equation (19), equal to 

accepted equating of the experimental quenching constant 
valid only if
The value of K can be estimated from the dependence of 

The 
3. Conclusion
It is concluded then that introducing the above correction may be a key moment in obtaining the true values of
the association constants. The relation 
quantum yield. In this case, the high concentrations of 
necessitates the introduction of a corrective factor which is also obligatory for the case of large complexing constants.
Cite this paper
Nikolay L.Lavrik,Nikolay M.Bazhin, (2014) On the Question of Defining the Association Constants by the Method of Fluorescence Quenching. American Journal of Analytical Chemistry,05,1065-1068. doi: 10.4236/ajac.2014.516113
References
- 1. De Weert, M. and Stella, L. (2011) Fluorescence Quenching and Ligand Binding: A Critical Discussion of a Popular Methodology. Journal of Molecular Structure, 998, 144-150.
http://dx.doi.org/10.1016/j.molstruc.2011.05.023 - 2. Lakovicz, J. (2010) Principles of Fluorescence Spectroscopy. 3rd Edition, Springer, Berlin.



