Open Journal of Composite Materials
Vol.05 No.01(2015), Article ID:52630,5 pages
10.4236/ojcm.2015.51002
FTIR and Raman Studies of Cellulose Fibers of Luffa cylindrica
Chhatrapati Parida1, Sarat Kumar Dash2, Chinmay Pradhan3
1Depatment of Physics, Orissa University of Agriculture and Technology, Bhubaneswar, India
2Department of Education in Science and Mathematics, National Council of Educational Research and Training, Bhubaneswar, India
3Post Graduate Department of Botany, Vani Vihar, Utkal University, Bhubaneswar, India
Email: sivaji_1976@yahoo.co.in, skdash59@yahoo.com, chinmay-pr@yahoo.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 25 October 2014; revised 30 November 2014; accepted 15 December 2014
ABSTRACT
In view of biomedical applications of cellulose fibers in orthopedics, dentistry and reconstructive surgery, Luffa cylindrica (LC), a local forest product of Orissa, India, has been used for preparation of alkali treated LC fiber modified with calcium carbonate and calcium phosphate separately by following standard procedures. FTIR and Raman spectra were obtained for these samples at wavelength range 500 - 4000 cm−1 and 300 - 3000 cm−1 respectively. Lattice structures of cellulose i.e., crystalline cellulose and amorphous cellulose were detected using Raman spectroscopy and discussed. The property of cellulose such as its degree of crystallinity was determined from intensity of FT IR peaks and was found to be 74.12%. The presence of calcite and hydroxy apatite, polymorphs of calcium carbonate and calcium phosphate respectively were confirmed in the treated modified LC fibers which can be used as bioactive materials.
Keywords:
Degree of Crystallinity, Hydroxy Apatite, FTIR, LC Fibers, Raman Spectra

1. Introduction
At present, it is a challenge before the researchers to develop new materials for biomedical applications. Calcium phosphate based bio-materials such as hydroxy apatite (Hap) and β tri calcium phosphate (β tcp) are extensively used for bone replacement, dental filling, bone tissue engineering, drug delivery, etc. [1] [2] .
Morpho synthesis is an important aspect in the development of new materials in many fields. Such process is used in chemical construction and patterning of inorganic materials with unusual and complex architecture over the surface of any material [3] . Many need based inorganic materials having specific morphology can be tailored artificially using this route. Simon R. Hall et al. in 2003 [4] described an exceedingly facile technique for replicating the complex surface morphology of flower and tree pollen grains. Cook G. et al. in 2003 [5] reported coating of silica on the wings of butterfly. Yang L et al. in 2010 [6] applied calcium phosphate ceramic coatings on surfaces of metallic and polymeric biomaterials. Such coating can improve its performance in bone repair and regeneration.
Infrared spectroscopy is nowadays one of the widely used analytical techniques. It is well known that the main constituent in natural fiber is the cellulose which acts as the reinforcing material in the cell wall. The cellulose molecules are held in micro fibrils in which there is extensive hydrogen bonding between cellulose chains, producing a strong crystalline structure. Much work has been reported [7] [8] on the characterization of the hydrogen bonds in cellulose by using various techniques, among which FTIR has been proved to be one of the most useful method. Cellulose occurs in the form of long, slender chains, polymer of glucopyranose units. Hydroxyl groups in each unit contribute to the formation of various kinds of inter and intra-molecular hydrogen bonds. The intermolecular H-bonding is between each glucopyranose unit and the intramolecular bonding is between -OH group of fiber and -OH group of matrix. The formation of inter and intra-molecular hydrogen bonds in the cellulose not only has a strong influence on the physical properties of cellulose, including solubility, hydroxyl reactivity and crystallinity, but also plays an important role in the mechanical properties of the cellulose based composites. Among other properties of cellulose, crystallinity of cellulose plays a significant effect on physical, chemical and mechanical properties of cellulose based composites. With increase in crystallinity of cellulose, the properties like tensile strength, compressive strength, storage modulus, thermal stability of composites, etc. can be enhanced. The crystallinity of cellulose in treated LC fiber was determined by comparing the intensity peaks from FTIR spectra at 1454 cm−1 and 893 cm−1 [8] as given by
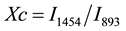
where Xc is the degree of crystallinity of cellulose and I1454 and I893 are the intensity of FTIR peak at 1454 cm−1 and 893 cm−1 respectively.
In a manner similar to FTIR spectroscopy, Raman spectroscopy is based on the existence of a spectrum of normal vibration modes in a given molecular compound. In traditional FTIR spectroscopy the intensity of transmitted radiation was studied whereas in Raman spectroscopy the intensity of scattered radiation is studied. Raman spectrum is generated by directing a laser onto the sample and observing the patterns of light waves which are scattered at higher and lower wavelengths relative to that of the incident laser beam. The shift provides information about vibration, rotational and other low frequency transitions in the given molecules. Therefore vibration spectroscopy like Raman spectroscopy plays an important role in the investigation of cellulose and cellulose based structures. The studies made by many researchers [9] [10] have confirmed the advantages of this analytical method over FTIR and NMR spectroscopy for determining the molecular conformations and hydrogen bonding patterns of cellulose and cellulose based materials. The main advantage of Raman spectra is the use of red excitation laser beam. The lasers avoid fluorescence of the cellulose based materials.
In our research, compounds of calcium carbonate and calcium phosphate are artificially synthesized over the surface of LC fibers. Presence of polymorphs of calcium phosphate such as hydroxy apatite and β tri calcium phosphate on the surface of LC fibers are confirmed from Raman spectroscopy. It is interesting to note that the crystallinity index of treated LC fiber was determined from FT IR spectral analysis and it was found to be 74.71%. The high % of crystallinity of cellulose in treated LC fiber was an important result to be considered in this study. The objective of our investigation is to transform the low priced, readily available agricultural waste, i.e. fruit of LC into a high value product. This work may subsequently demonstrate the effectiveness of cellulose nano crystals derived from LC in improving the mechanical, thermal and electrical properties of matrix polymer.
2. Methodology
The fruit of LC was collected from local forest area near Barang, Orissa, India. The chemicals such as calcium chloride (CaCl2 2H2O, 97%), sodium carbonate (Na2CO3, 95%), di sodium hydrogen phosphate (Na2HPO4 2H2O, 99.5%), all of AR grade were procured from E. Merck, India.
The fibers of LC were cut into small pieces of length around 2 cm. These were washed thoroughly with deionized water to remove impurities like oil, dust, part of leaf, etc. These were then dried at 70˚C in vacuum oven for 20 minutes. The dried LC fibers are subjected to chemical treatment such as treatment with alkali followed by bleaching and acid hydrolysis, For alkali treatment, the LC fibers were soaked in a 5% NaOH solution at 80˚C for 1 h. The soaked LC fibers were then washed with fresh water for 30 minutes to remove any excess NaOH sticking to the surface of LC fiber. The fibers were then dried at room temperature for 48 h followed by drying in oven at 60˚C for 6 h. During alkali treatment given to the natural fibers the hemicelluloses and lignin present in the natural fibers are extracted. In this way the number of -OH groups present in the fiber is reduced. The decrease in -OH groups increases hydrophobicity of natural fibers which strengthen the bonding between fiber and matrix. There is disruption of hydrogen bonds in the network structure of cellulose due to the alkali treatment. Thus it increases the surface roughness and the adhesion between fiber and matrix. This treatment depolymerizes cellulose and exposes the short length crystallites of cellulose. The alkali treated LC fibers were bleached with 2% sodium hypochlorite solution. The mixture was continuously stirred for 2 h at 80˚C. After this the mixture was poured through a filter paper in a funnel. The solid fibers were trapped by the filter paper. After filtration the fibers were washed with distilled water till neutral pH was obtained. The pulp obtained after bleaching is termed usually as micro crystalline cellulose (MCC). The colour of the bleached LC fibers appear yellowish from black. The bleached LC fiber/water suspension was prepared and kept on an ice bath. H2SO4 was added slowly under continuous stirring to the suspension placed in an ice water bath, until the final concentration of 60% H2SO4 was reached. The obtained suspension was then heated at 45˚C under continuous stirring for 2 h. In order to remove excess acid the mixture was washed and centrifuged using an ultracentrifuge at 30˚C for 20 minutes with 7000 rpm.
The treated LC fiber were then immersed in CaCl2 solution for 12 h at room temperature and then washed with distilled water for removing excess calcium deposited on it. Now, the LC fiber modified with CaCl2, were re-immersed in Na2HPO4 solution for 12 h at room temperature to deposit compounds of calcium phosphate over it. Another set of the LC fiber modified with CaCl2 were reimmersed in Na2Co3 solution for 12 h to deposit compounds of calcium carbonate on its surface. Thus two separate sets of modified LC fibers were prepared. One set of treated LC fibers are modified by calcium carbonate. The other set of treated LC fibers are modified with calcium phosphate. After 12 h the modified LC fibers were washed again with distilled water.
A mixture of 5.0 mg of dried sample and 200 mg of KBr was pressed into a disk for FTIR measurement. The amount of mixture was kept constant to obtain repeatable transmission from the sample. KBr pellet is used because KBr does not absorb in the IR wavelengths between 4000 - 400 wave numbers (cm−1) and in this way the material could be easily dispersed. All the samples were examined by FTIR spectroscopy (FTIR Nicolet 6700/ Thermofisher Scientific) using KBr pellet technique in the region 500 cm−1 - 4000 cm−1 to identify the functional groups in the samples. A Raman spectrum is generated by directing a laser beam onto the sample and observing the patterns of light waves which are scattered at higher and lower wavelengths relative to that of the incident laser beam. Raman measurements were performed with a HORIBA Jobin Yvon, France Lab RAM HR 800 apparatus. A He-Ne laser source of 632 nm was used. The laser spot diameter was about 1 μm and spectral resolution was 2 cm−1. The measurements were performed between 300 cm−1 and 3000 cm−1.
3. Results and Discussion
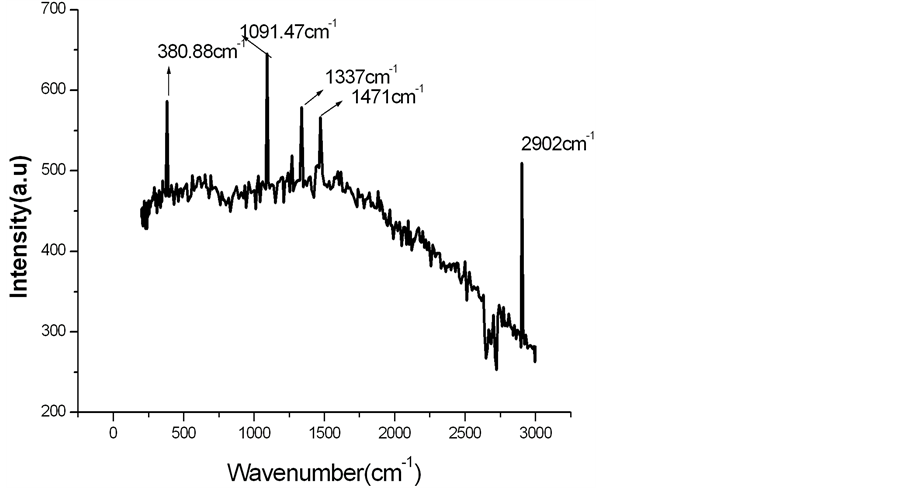
The Raman spectra of alkali treated LC fiber are shown in Figure 1.
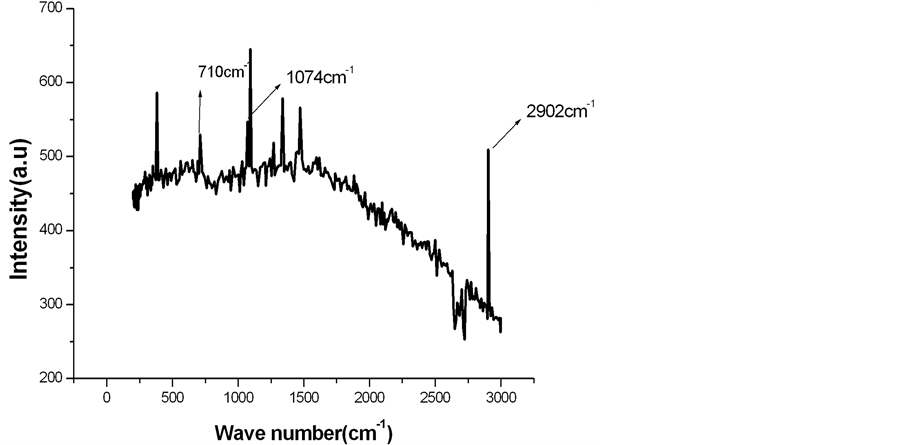
Infrared (IR) radiations normally alters the electric dipole moment of the molecule, on the other hand Raman spectroscopy is dependent on electric polarizability. Raman spectroscopy measures the vibrational modes of non polar bands. Non polar functional groups give rise to a strong band in Raman spectra where as polar functional groups give rise to strong IR bands. The polar -OH groups present in LC fibers are strongly reflected in IR bands at around 3400 cm−1. However in Figure 1 there is no intensity for polar -OH groups in the Raman spectra at 3400 cm−1. The characteristic peak at 2902 cm−1 in Figure 1 shows C-H, CH2 stretching of cellulose. The peaks at 1471 cm−1 indicates H-C-H bending, the peak at 1337 cm−1 represents H-O-C bending. The modes at 1091 cm−1 are mainly attributed to the β-1,4-glycosidic linkages of the glucopyranose units of cellulose. However, the peak at 380.88 cm−1 is the characteristic of amorphous cellulose [9] [10] . The treated LC fibers are modified with calcium phosphate and calcium carbonate. The modified form of treated LC fibers is characterized by Raman spectroscopy. The Raman spectra of alkali treated LC fiber modified by calcium carbonate are shown in Figure 2.
The additional peak observed at 710 cm−1 and 1074 cm−1 in Figure 2 in comparison to the peaks in Figure 1
Figure 1. Raman spectra of alkali treated LC fiber.
Figure 2. Raman spectra of alkali treated LC fiber modified by calcium carbonate.
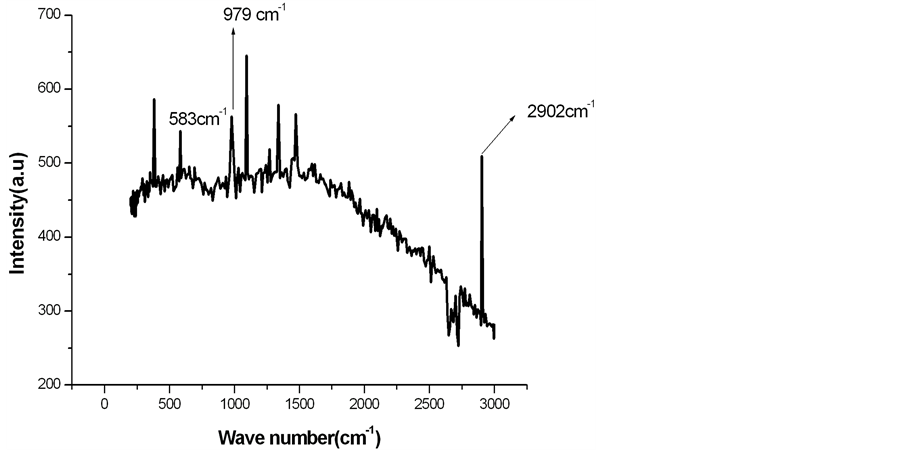
is characteristic of calcite, which is a polymorph of calcium carbonate [11] . The Raman spectra of alkali treated LC fiber modified by calcium phosphate are given in Figure 3.
Figure 3 shows the presence of an additional peak at 583 cm−1 and 979 cm−1 in comparison to that of the peaks as obtained in Figure 1 are characteristics of hydroxy apatite, which is a polymorph of calcium phosphate. The peak at 979 cm−1 in Figure 3 corresponds to stretching mode of tetrahedral phosphate ions in hydroxy apatite [11] . However, all the Raman peaks obtained are displayed in tabular form in Table 1.
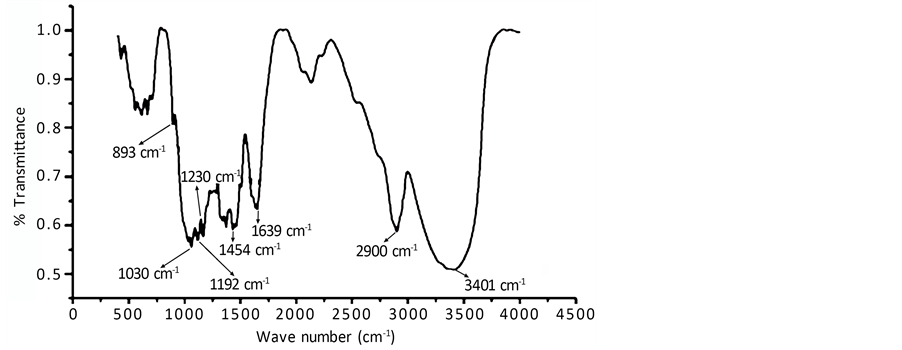
Figure 4 shows the FTIR spectrum of alkali treated LC fiber from wave number 400 cm−1 to 4000 cm−1.
A close perusal of Figure 4 shows that 3401 cm−1 band is due to the H-bonded H-O stretching and 2900 cm−1 band is due to the C-H stretching in methyl and methylene group. Normally the band 1735 cm−1 in IR spectra of natural fiber is assigned to C-O stretching of carboxyl group of hemicellulose present in the fiber. But such band
Figure 3. Raman spectra of alkali treated LC fiber modified by calcium phosphate.
Table 1. Characteristic frequencies from Raman spectra.
Figure 4. FTIR spectrum of alkali treated LC fiber.
is absent in Figure 4. It indicates removal of hemicellulose from the LC fiber after alkali treatment. The band 1639 cm−1 is ascribed to the O-H bending of absorbed water or moisture. The small peak at 1454 cm−1 is attributed to asymmetric CH3 deformation in lignin. The band at 1454 cm−1 is also assigned to CH2 bending. There exists a doublet band at 1192 cm−1 and 1230 cm−1 in Figure 4. This doublet bands are characteristics of alkali treated fiber or mercerized cellulose [7] . The band at 893 cm−1 may be an indication of symmetric in-phase stretching mode in lignin. The small kink at 893 cm−1 probably indicates partial removal of lignin during alkali treatment [9] [10] . Figure 4 was also used for determination of crystallinity index of cellulose fibers. The intensity (% transmittance) at 1454 cm−1 and at 893 cm−1 was found to be 0.59977 and 0.80841 respectively. The crystallinity was found to be 74.71%.
4. Conclusion
Alkali treated LC fiber modified with calcium phosphate and calcium carbonate was prepared and analyzed by using Raman and FTIR spectra for phase identification. From Raman spectra, the presence of polymorphs of calcium carbonate and calcium phosphate was confirmed. The peak at 979 cm−1 indicates presence of hydroxy apatite. Hydroxy apatite is a polymorph of calcium phosphate having chemical formula Ca10 (PO4)6 (OH)2 which is an important bio mineral. It is chemically similar to the mineral component of bone and hard tissues in mammals. It is one of few materials that are classed as bioactive, meaning that it will support bone in growth and Osseo integration when used in orthopedic, dental and maxillofacial applications [1] [2] [12] . From FTIR spectra, the crystallinity index of treated LC fiber was found to be 74.71%. Therefore, cellulose fibers modified by calcium phosphate and calcium carbonate can be used as reinforcing phase in nanocomposites. Such composites can explore various ways of utilizing the inherently high modulus and strength of cellulose fibers. The presence of Ca salts in cellulose fibers opens the possibility of using cellulose fiber reinforced composite in various biomedical applications.
References
- Kumar, G.S., Girija, E.K., Thamizhavel, A., Yokogawa, Y. and Kalkura, S.N. (2010) Synthesis and characterization of Bioactive Hydroxyapatite-Calcite Nanocomposite for Biomedical Applications. Journal of Colloid and Interface Sci- ence, 349, 56-62.
- Preve, P.S. (2000) X-Ray Diffraction Characterization of Crystallinityu and Phase Composition in Plasma Sprayed Hydroxyl Apatite Coatings. Journal of Thermal Spray Technology, 9, 369-376.
- Walsh, D., Lebeau, B. and Mann, S, (1999) Morphosynthesis of Calcium Carbonate (Vaterite) Microsponges. Advanced Materials, 11, 324-328.
- Hall, S.R., Bolger, H. and Mann, S. (2003) Morphosynthesis of Complex Inorganic Forms Using Pollen Grain Templates. Chemical Communications, 22, 2784-2785.
- Cook, G., Timms, P.L. and Spickermann, C.G. (2003) Exact Replication of Biological Structures by Chemical Vapor Deposition of Silica. Angewandte Chemie International Edition, 42, 557-559. http://dx.doi.org/10.1002/anie.200390160
- Yang, L., Perez-Amodio, S., Barrère-de Groot, F.Y., Everts, V., van Blitterswijk, C.A. and Habibovic, P. (2010) The Effects of Inorganic Additives to Calcium Phosphate on in Vitro Behavior of Osteoblasts and Osteoclasts. Biomaterials, 31, 2976-2989.
- Herrea-Franco, P.J. and Valadez-Gonzalez, A. (2005) A Study of the Mechanical Properties of Short Natural-Fiber Re- inforced Composites. Composites Part B, 36, 597-608.
- Ciolain, D., Ciolain, F. and Popa, V.I. (2011) Amorphous Cellulose―Structure and Characterization. Cellulose Chemistry and Technology, 45, 13-21.
- Schenzel, K. and Fischer, S. (2004) Applications of FT Raman for the Characterization of Cellulose. Lenzinzer Beri- chte, 83, 64-70.
- Li, L.C. (2007) Identification of Textile Fiber by Raman Microspectroscopy. Forensic Science Journal, 6, 55-62.
- Mazali, I.O. and Alves, O.L. (2005) Morphosynthesis: High Fidelity Inorganic Replica of the Fibrous Network of Luffa cylindrica. Annals of the Brazilian Academy of Sciences, 77, 25-31.
- D.K. Pattanyak, Divya P, Sujal Upadhaya, (2005) Synthesis and Evolution of Hydroxyl Apatite Ceramics. Trends in Biomaterials and Artificial Organs, 18, 169-175.