Journal of Crystallization Process and Technology
Vol.4 No.3(2014), Article ID:47515,5 pages
DOI:10.4236/jcpt.2014.43018
Synthesis and X-Ray Structure of Important Anticancer Nucleosides Intermediate (2R,3S,4S,5R)-2-(acetoxymethyl)-5- (3-bromo-5-(methoxycarbonyl)- 1H-1,2,4-triazol-1-yl)tetrahydrofuran- 3,4-diyl Diacetate
Yang Liu1*, Guanghui Tian1, Hongguang Ge1, Xiaoyan Cao1, Daihua Hu1, Dehua Zhang2
1School of Chemistry and Environmental Science, Shaanxi University of Technology, Hanzhong, China
2Department of Chemistry and Environmental Engineering, Hubei Normal University, Huangshi, China
Email: *liuyang@snut.edu.cn
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 17 April 2014; revised 17 May 2014; accepted 17 June 2014
ABSTRACT
An important anticancer nucleosides intermediate (2R,3S,4S,5R)-2-(acetoxymethyl)-5-(3-bromo- 5-(methoxycar-bonyl)-1H-1,2,4-triazol-1-yl)tetrahydrofuran-3,4-diyl diacetate was synthesized by directly coupling the bromotriazole with the protected ribose sugar, and have given the corresponding product in moderate yield. Its structure and conformation were confirmed by single crystal X-ray diffraction.
Keywords: Anticancer Nucleosides, Intermediate, Triazole Nucleosides

1. Introduction
Synthetic nucleoside mimics with modified nucleobase and/or sugar moieties are of considerable importance in the search for promising candidates leads endowed with biologically interesting activity [1] . Some well-known nucleoside drugs consist of antiviral drugs ribavirin, acyclovir and zidovudine, as well as the anticancer drugs gemcitabine and cladribine. These nucleoside analogs are able to mimic natural nucleosides and as such serve as building units or inhibitors to interfere in nucleic acid synthesis or block the biological processes involving the action of nucleos(t)ides [2] -[5] . Then, they can inhibit replicating viruses and uncontrolable cancer cell proliferation, leading to potent and effective antiviral and anticancer activity, respectively. Based on the rationale to combine the special features of unnatural triazole heterocycles with those of the appended aromatic groups on the nucleobases, we have been engaged in sythesizing structurally diverse triazole nucleoside analogs bearing aromatic moieties on the triazole nucleobase with the view of identifying new structural leads exhibiting antiviral and anticancer activity. The synthesis of these nucleosides was achieved via transition metal catalyzed modern synthetic reactions, such as Suzuki coupling [6] , sonogashira reaction [7] , C-N coupling [8] and C-S coupling [9] , starting with readily available bromotriazole nucleosides intermediate, and the synthetic procedure developed is simple and easy to perform.
We report on the synthesis of an important anticancer nucleosides intermediate, using a simple and efficient condendsation procedure, giving the corresponding products in moderate yield. The intermediate can be further exploited for the synthesis of novel structural nucleoside analogues, which are currently a class of extremely important compounds in the search for anticancer drug candidates.
The synthesis of the title anticancer nucleosides intermediate 1 is shown in Scheme 1. The structures and conformations of compound 1 were further elucidated by their single crystal X-ray diffraction, as shown in Figure 1. Three-dimensional molecular-packing diagram of the title compound was shown in Figure 2.
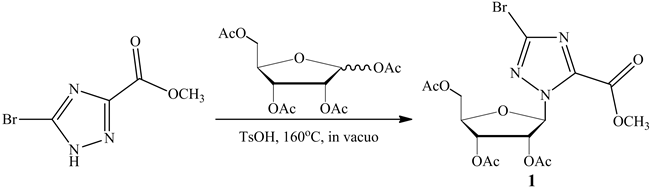
Scheme 1. Synthesis of the anticancer nucleosides intermediate 1.
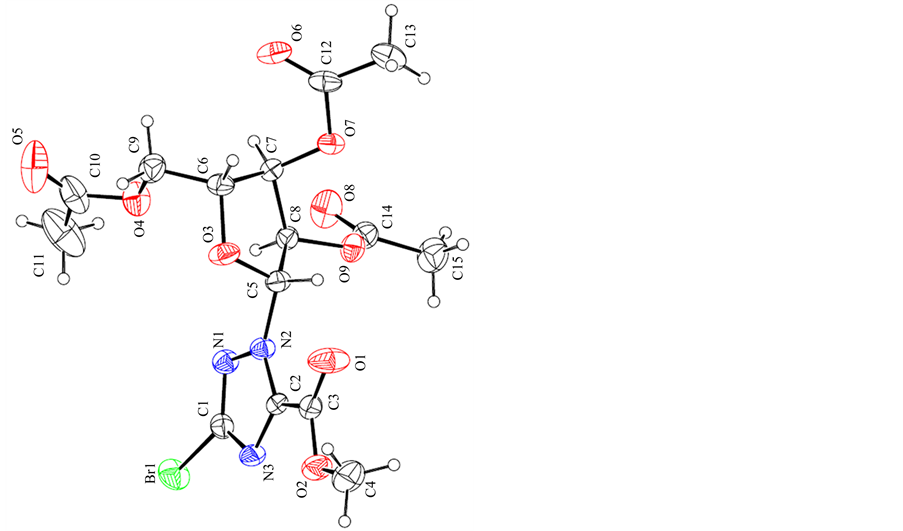
Figure 1. The single crystal structure of compound 1.

Figure 2. Three-dimensional molecular-packing diagram of compound 1.
The crystals of 1 were obtained by slow evaporation of their solution in ethyl acetate/petroleum ether (3:1, v/v) mixtures. The crystal structures of 1 clearly revealed that it has well-defined geometry due to the rigidity that the fused rings confer on the molecule. Its structures and conformations were confirmed by single crystal X-ray diffraction. The molecular conformation is stabilized by N---H...O hydrogen bond; the crystal packing is governed by C---H...O and C---H...N hydrogen interactions resulting in a three-dimensional network. These values are suitable for the complexation of an aromatic ring by p-p stacking interactions.
2. Experimental Details
2.1. Preparation of 1
A mixture of methyl 5-bromo-1H-1,2,4-triazole-3-carboxylate (0.936 g, 4.55 mmol) and 1,2,3,5-tetra-O-acetyl- β-D-ribofuranose (1.45 g, 4.55 mmol) was heated to 160˚C in the presence of p-methyl benzenesulfonic acid (10 mg) and kept under reduced pressure for 15 min. The resulting mixture was separated by FC (silica gel, CH2Cl2/ acetone 30:1). The purified material was dried in vacuo to afford the corresponding product as white solid, an amount of 0.95 g of compound was obtained with a yield of 45%.
White crystals suitable for XRD formed after a few days of slow evaporation of the solvent at room temperature over several days. White single crystals of the title compound are shown in Figure 1. The crystal structures of 1 clearly revealed that it has well-defined geometry due to the rigidity that the fused rings confer on the molecule.
2.2. Characterization
All reagents obtained from commercial sources were of AR grade. Melting points were determined with XT4A micromelting point apparatus and were uncorrected. The 1H NMR was recorded on a Mercury Plus-400 spectrometer with TMS as internal reference and CDCl3 as solvent. IR were recorded on a Perkin-Elmer PE-983 IR spectrometer as KBr pellets with absorption in cm−1. MS were obtained with Finnigan Trace MS instrument using EI method. Elemental analyses were carried out on a Vario EL III instrument.
3. Results and Discussion
3.1. Single Crystal X-Ray Diffraction Analysis
Single crystal X-ray diffraction studies were carried out on the grown crystals. The X-ray date was collected using X-ray diffractometer (Model: Bruker Smart APEX-CCD). A white crystal of the title compound 1 was each mounted on a glass fibre in a random orientation at 298(2) K. The determination of the unit cell and the data collection were performed with MoKa radiation (l = 0.71073 Å) on a Bruker Smart Apex-CCD diffactometer with a y-w scan mode. The structure was solved by direct methods with SHELXS-97 program and expanded by Fourier technique. The non-hydrogen atoms were refined anisotropically, and the hydrogen atoms were placed at the calculated positions.
Crystal data for 1: C15H18BrN3O9, M = 464.22, Triclinic, space group P2(1), a = 8.785 Å, b = 11.613 Å, c = 9.713 Å, α = 90.00˚, β = 95.04˚, γ = 90.00˚, V = 987.1 Å3, Z = 2, Dc = 1.562 Mg/m3. Reflections collected: 8324, independent reflections: 4404 [Rint = 0.]">0311], Final R indices [I > 2 sigma(I)]: R1 = 0.0556, wR2 = 0.1023. R indices (all data): R1 = 0.0760, wR2 = 0.1111.
3.2. The Structure Characterized
Compound 1
1H NMR (300 MHz, CDCl3): 6.90 (d, 1H, J = 3.0 Hz, H-1’), 5.80 - 5.83 (m, 1H, H-2’), 5.67 - 5.72 (m, 1H, H-3’), 4.42 - 4.48 (m, 2H, H-4’ + H-5’), 4.12 - 4.20 (m, 1H, H-5’), 4.02 (s, 3H, -OCH3), 2.14 (s, 3H, -C(O)CH3), 2.12 (s, 3H, -C(O)CH3), 2.08 (s, 3H, -C(O)CH3), MS: m/z 465.2 [M + H]+.
13C-NMR (CDCl3): 170.0 (CO); 169.0 (CO); 168.9 (CO); 156.5 (CO); 145.8 (C(5)); 140.2 (C(3)); 89.7 (C(1’)); 81.2 (C(2’)); 74.7 (C(3’)); 71.1 (C(4’)); 63.1 (C(5’)); 54.3 (MeO); 21.7 (Me); 21.4 (2 Me). FAB-MS: 464 (M+, C15H18BrN3O9), 466 ([M + 2]+).
4. Conclusion
A intermediate bromotriazole nucleoside (2R,3S,4S,5R)-2-(acetoxymethyl)-5-(3-bromo-5-(methoxycarbonyl)- 1H-1,2,4-triazol-1-yl)tetrahydrofuran-3,4-diyl diacetate has been synthesised. The structure and conformation were confirmed by single crystal X-ray diffraction and 1H NMR. Novel triazole nucleoside analogs bearing aromatic systems on the nucleobase have been synthesised from this intermediate. They have great potential to bind aromatic guest molecules. Further studies on their binding properties are in progress.
Acknowledgements
We thank the Shaanxi provincial Department of Education (Grant No. 2013JK0659) and Shaanxi University of Technology (SLGQD13-2) for financial support, the Hubei provincial Department of Education (Grant No. D20112503), Hubei Key Laboratory of Pollutant Analysis & Reuse Technology (Grant No. KL2013G06).
References
- Claire, S. (2001) Nucleoside Mimetics: Their Chemistry and Biological Properties. Gordon and Beach Science, UK.
- De Clercq, E. (2007) The Design of Drugs for HIV and HCV. Nature Reviews Drug Discovery, 6, 1001-1018. .http://dx.doi.org/10.1038/nrd2424
- Galmarini, C.M., Popowycz, F. and Joseph, B. (2008) Cytotoxic Nucleoside Analogues: Different Strategies to Improve Their Clinical Efficacy. Current Medicinal Chemistry, 15, 1072-1082..http://dx.doi.org/10.2174/092986708784221449
- Galmarini, C.M., Mackey, J.R. and Dumontet, C. (2002) Nucleoside Analogues and Nucleobases in Cancer Treatment. The Lancet Oncology, 3, 415-424. http://dx.doi.org/10.1016/S1470-2045(02)00788-X
- Berdis, A.J. (2008) DNA Polymerases as Therapeutic Targets. Biochemistry, 47, 8253-8260..http://dx.doi.org/10.1021/bi801179f
- Wan, J.Q., Zhu, R.Z., Xia, Y., Qu, F.Q., Wu, Q.Y., Yang, G.F., Neyts, J. and Peng, L. (2006) Synthesis of 5-Aryltriazole Ribonucleosides via Suzuki Coupling and Promoted by Microwave Irradiation. Tetrahedron Letters, 47, 6727-6731. http://dx.doi.org/10.1016/j.tetlet.2006.07.103
- Xia, Y., Liu, Y., Wan, J.Q., Wang, M.H., Rocchi, P., Qu, F.Q., Iovanna, J.L. and Peng, L. (2009) Novel Triazole Ribonucleoside Down-Regulates Heat Shock Protein 27 and Induces Potent Anticancer Activity on Drug-Resistant Pancreatic Cancer. Journal of Medicinal Chemistry, 52, 6083-6096. http://dx.doi.org/10.1021/jm900960v
- Fan, Y.T., Xia, Y., Tang, J.J., Ziarelli, F., Qu, F.Q., Rocchi, P., Iovanna, J.L. and Peng, L. (2012) An Efficient Mixed-Ligand Pd Catalytic System to Promote C-N Coupling for the Synthesis of N-Arylaminotriazole Nucleosides. Chemistry—A European Journal, 18, 2221-2225. http://dx.doi.org/10.1002/chem.201103918
- Cong, M., Fan, Y.T., Raimundo, J.-M., Xia, Y., Liu, Y., Quelever, G., Qu, F.Q. and Peng, L. (2013) C-S Coupling Using a Mixed-Ligand Pd Catalyst: A Highly Effective Strategy for Synthesizing Arylthio-Substituted Heterocycles. Chemistry—A European Journal, 19, 17267-17272. http://dx.doi.org/10.1002/chem.201302174
NOTES

*Corresponding author.

