Advances in Bioscience and Biotechnology
Vol. 4 No. 12 (2013) , Article ID: 41360 , 8 pages DOI:10.4236/abb.2013.412151
Probiotic levels, chemical composition and fermentative characteristics in solid state fermentation of paper sludge for animal feeding
![]()
1Faculty of Zootechnology and Ecology, Autonomous University of Chihuahua, Chihuahua, México 2Department of Biomedical Sciences, Autonomous University of Juárez City Campus, Nuevo Casas Grandes, Mexico
3Faculty of Veterinary Medicine and Zootechnology, Autonomous University of Tamaulipas, Ciudad Victoria, Mexico
Email: *ycastillo75@yahoo.com
Copyright © 2013 Oscar Ruiz-Barrera et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 10 September 2013; revised 12 November 2013; accepted 11 December 2013
Keywords: Fermentation; Paper Sludge; Yeast; Chemical Composition
ABSTRACT
The sludge paper of the industry treated with probiotics in solid state fermentation (SSF) could be used as ingredient in rations for animal feeding. This study assessed the effect of four probiotic (Prozoot15®) levels (PT) on chemical and fermentative characteristics in SSF of the paper sludge (PS) at controlled temperature (30˚C) in laboratory scale. The tested treatments (T) were: T1 (0% PS), T2 (50 g/kg PS), T3 (100 g/kg PS) and T4 (150 g/kg PS), which were fermented at 0, 24, 48 and 72 h, according to a completely randomized design, in a 4 × 4 factorial arrangement with six repetitions per sampling. All treatments included (g/kg DM) 300 molasses, 15 urea, 20 ammonium sulfate, 9 calcium carbonate and 5 of vitamin and mineral premix, plus the PS which was substituted by the PT at 0, 50, 100 and 150 g/kg DM. The results showed a decrease in pH in all treatments at 24 h; however the lowest pH was at 72 h of fermentation. At 72 h of fermentation, the PT addition in T4 increased crude protein, true protein and yeast counts (P < 0.05), and decreased pH (P < 0.05). In all fermentation time, the PT addition increased ether extract, lactic acid and ammonia nitrogen (P < 0.05) and decreased dry matter, ash, NDF and ADF (P < 0.05). It was concluded that the addition of 150 g/kg of PT in SSF of paper sludge improves crude and true protein, ether extract, lactic acid, and ammonia. This treatment may have potential use in animal feeding.
1. INTRODUCTION
It is known that urban industries pollute the environment by dumping their waste, and causing contamination of soil and water (surface and underground). During the industrial processing of paper in Chihuahua City (Northern Mexico), the solid residue known as sludge of paper is produced; the production of this by-product is about 120 tons per day, which could be used as energy source in the ruminant feeding without generating pollution [1]. Due to the increasing amount of paper sludge generated in this industry, it is necessary to find alternatives to use it. The sludge of paper is characterized by its high structural carbohydrates content, low-protein, and high dry matter that contains inorganic matter [2]; these nutrients may be useful in ruminant diets.
For the potential use of this by-product, other components, like probiotics, are necessary to improve their nutritional value. Probiotics are live micro-organisms cultures, which are administered in adequate quantities, confer beneficial effects to the health of host, and are a natural alternative to improve the animal metabolism [3]. In addition, microbial cultures may improve the nutritional characteristics of by-products when they are treated in solid state fermentation. In this process, the production of organic acids, dry matter digestibility, and protein content are increased; also, there is a decrease in pH and cell wall fractions [4].
Together, the sludge of paper and probiotics could be a part of the diet and used as ingredients in the animal production systems. Also, the sludge of paper and probiotics could be treated in a solid state fermentation processes (SSF). This process could improve the nutritional characteristics of the solid waste of paper industry. [5] defines the SSF as the culture of microorganisms in a particular substrate, allowing its growth and metabolism at proper temperature and aeration; this permits the use of microbial production or single-cell protein. In this sense, [6] mentions that the SSF consists in the growth of microorganisms on solid substrates in absence of free water; this improves the chemical composition of some by-products and generates new alternatives for animal feeding. However, there is not enough information on the fermentation in solid state of paper sludge with the addition of probiotics and considering its potential use in ruminant feeding. Thus, the objective of present study was to evaluate the effect of probiotic level on the fermentative characteristics and chemical composition of sludge paper as substrate in solid state fermentation. These results may show the potential use as a protein and rich ingredient of energy for animal feeding of the product obtained in SSF of paper sludge treated with probiotics. In addition to the possible reduction in feeding cost of livestock, it also contributes to the alleviation of the pollution effects produced by the paper industries.
2. MATERIALS AND METHODS
2.1. Treatments
The experiment was conducted in a laboratory-scale, using a completely randomized design with a 4 × 4 factorial arrangement, using six repetitions per sampling period. It was considered the effect of four probiotic levels testing the following treatments: T1 control; T2 50 g/kg PS; T3 100 g/kg PS; T4 150 g/kg PS, which were considered and defined as factor A. Four times of fermentation (0, 24, 48, and 72 h) were considered as factor B.
2.2. Experimental Procedure
The paper sludge was obtained from a commercial factory that processes paper in Chihuahua City. The sludge of paper was mixed (g/kg) with 300 molasses; 15 urea; 2 ammonium sulfate; 5 premix of vitamins and minerals; 9 calcium carbonate and the probiotic (amount according to treatment). The probiotic used in this experiment is a mexican commercial trademark (Prozoot15®) rich in yeasts and lactobacilli, organic acids and vitamins. For the fermentation were used 150 g of the different experimental mixtures, which were deposited in glass bottles of 250 ml in capacity that previously were sterilized, which were covered at top with cotton and placed in an incubator with shaker at a controlled temperature (30˚C). Each glass bottle was considered as an experimental unit and six bottles per sampling period were used for each treatment (six repetitions). During the fermentation period were taken out six bottles per treatment at fermentation times of 0, 24, 48 and 72 h.
After the incubation periods, the content of bottles were fully collected and individually homogenized. Liquid samples were collected for laboratory analysis as follows: 5 g of the fermented mixture in each treatment, 20 ml of distilled water was added and mixed in a shaker (Eberbach) for 30 minutes. Subsequently, samples were filtered through sterile gauze to separate solids from liquids. For each sample several subsamples were taken for subsequent determinations. The supernatant was a used to determine the indicator of fermentation: pH, ammonia-N, lactic acid and yeast count. The remaining portion of each bottle was dried in an oven at 60˚C (THELCO), and ground in manual mill (ESTRELLA) for laboratory analysis (dry matter, mineral matter, ether extract, crude protein, true protein, neutral detergent fiber and acid detergent fiber).
2.3. Analysis
The supernatant of the fermented product, as described in the experimental procedure, was used for determination of pH with a table potentiometer (HANNA), lactic acid (LA) according to the procedure described by [7], yeast counts (YC) according with the methodology described by [8], ammonia nitrogen (N-NH3) according to the colorimetric method described by [9].
The fermented product, previously dried and ground, was used for the determination of matter dry (DM), ash, ether extract (EE), crude protein (CP) and true protein (TP). These determinations were made employing the procedures of the [10]. Also were determined acid detergent fiber (ADF) and neutral detergent fiber (NDF), based on methodologies described by [11] and [12] respectively.
2.4. Statistical Analysis
The data from the variables were analyzed using the mixed procedures of [13]. The model included as fixed effects the treatment (probiotic levels), the fermentation times and the interaction probiotic level × fermentation time. The samples were considered as random effect. Values of P < 0.05 were declared significant.
3. RESULTS AND DISCUSSION
The results of chemical composition (DM, ash, EE, CP, TP, NDF and ADF) are shown in Table 1. There was an interaction effect of probiotic level and fermentation time (P < 0.05). The DM decreased in samples with the increase in PT level (P < 0.05). The lower values of DM
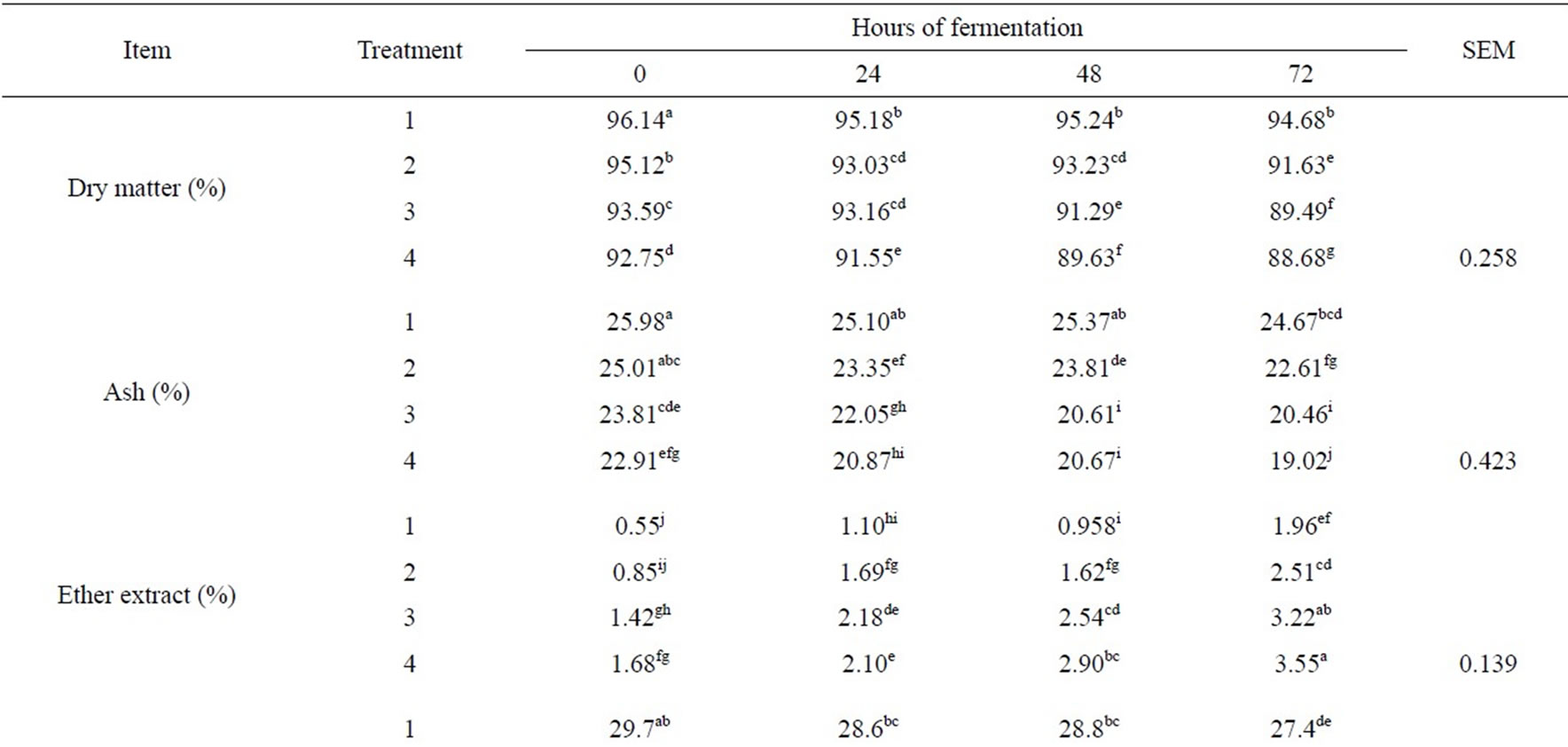

Table 1. Least square means of chemical composition of sludge paper in SSF with inclusion of different levels of probiotic in different hours of fermentation.
were in T3 at 72 h and in T4 at 48 and 72 h of fermentation. This decrease in DM content may be due to the use of structural and nonstructural carbohydrates as energy source for growth and survival of microorganisms. This effect also is observed with the inoculation of microorganisms in SSF of various substrates [8,14]. In agreement with present investigation, [15] obtained a decrease in DM content in SSF of rapeseed paste inoculated with a culture of Lactobacillus fermentum, Enterococcus faecium, Saccharomyces cerevisiae and Bacillus subtilis. This decrease in DM was probably caused by the consumption of carbohydrates. Also, [16] found a reduction in DM content due to the microbial growth in substrate, causing an increase of true protein.
The ash content decreased with the increase in probiotic level at all fermentation times (P < 0.05). The lower values of ash were observed in T3 and T4 at 48 and 72 hours of fermentation. This decrease in ash content could be due to the minerals use by the microbial population for protein synthesis; accordingly, the increase in probiotic addition could cause that the minerals in substrates were used for true protein production. These results are consistent with those obtained by [5]. These sense, [17] mentioned that the objective of the SSF is to increase the epiphyte microbial population using carbohydrates-rich substrates, urea as non-protein nitrogen and mineral premix.
The concentration of EE increased at all times of fermentation with the increase in PT level (P < 0.05). The highest values of EE were obtained in T3 and T4 at 72 h of fermentation. This increase in concentrations of EE could be the result of microbial growth, which caused further DM loss from the consumption of fermentable carbohydrates [18,19]. This sense, the microorganisms used carbohydrates, increasing the content of EE that was not consumed by them; this is consistent with [20] who obtained similar results with the fungus of Rhizopus oligosporus as inoculums in solid state fermentation of pearl millet. On the other hand, the EE increased with the fermentation time could be due to the existence of microorganisms such as yeasts and fungi that synthesized fats [21].
The NDF and ADF showed similar pattern (P < 0.05). The increase in PT level decreased the contents of these variables at all fermentation times. The lower values were in T3 at 72 h and in T4 at 48 and 72 h of fermentation. The fiber reduction probably was due to the degradation of the fibrous fraction by fibrolytic microorganisms; this is in agreement with [22] who used sugarcane as substrate in SSF, they found a reduction in cellulose and NDF level. Consistently, [23] found fibrolytic activity with three fungus species in SSF; they indicated that the reduction in fiber content was related to the presence of microorganisms responsible for fiber degradation. [24] observed that the treatment of lignocellulolytic substrates with fungi of the genus Pleurotus as inoculums in SSF caused changes in the chemical composition, reducing the fractions of NDF and ADF, cellulose and hemicelluloses. [25] mentioned that the effects of cellulose and hemicelluloses degradation are associated with the production of fibrolytic enzymes by microorganisms.
The CP content increased with the increase in probiotic level in all fermentation times (P < 0.05). The highest CP values were in T3 at 72 h and in T4 at 48 and 72 h of fermentation. In present study was observed that the probiotic addition to paper sludge in SSF increased the CP content. Accordingly, during the SSF processes has been observed an increase in CP of the substrate [18,26]. Consistently, [27] obtained increases in CP with different yeasts in SSF of apple bagasse. Also, [28] reported significant CP increases in SSF of oat and corn straws when used the fungus Phanaerochaete chrysosporium as inoculums; this was due to the transformation of sugars and non-protein nitrogen into CP. These results are consistent with those of [29] who found increases in CP using as inoculums yeast cultures of Candida utilis and the fungus Pleurotus ostreatus in SSF of the apple juice by product. These increases might be due to the multiplication of the epiphytic microorganisms that are responsible of the protein increase, which used soluble carbohydrates of high availability.
The TP also increased (P < 0.05) with the increase in PT level in all fermentation times. The highest values of TP were obtained in T3 and T4 at 48 and 72 hours of fermentation. The increase in TP content in probiotic treatments may have been related to the further development of microbial biomass [27]. This is consistent with [30] who reported that increase in TP was due to the increase in microbial biomass. Similarly, [31] concluded that TP increase may be related to the growth of microorganisms; also they mentioned that the TP could be an indirect method to measure the microbial growth in the processes of SSF.
The Table 2 shows the results of pH, LA, NH3 and cell counts (C). These fermentation indicators observed interaction effect of PT level and fermentation times (P < 0.05). The pH value decreased as the PT level increased in samples (P < 0.05); this decrease was observed from 24 to 72 h of fermentation. The lower pH values were in T3 and T4 at 48 and 72 h of fermentation. It was observed that in early stages of SSF, the pH was alkaline, but after, with the increase in fermentation time, it tended to become acid. Similar results were obtained by [32]. The alkalinity in the early fermentation stages may be related to the low concentration of organic acids, such as lactic acid, in the early stage of fermentation [31]. The pH declined at 24 h of fermentation, and probably was due to the increase in organic acids that were produced by the Lactobacilli species that increased in number of cells [34-36]. It is stated by [37] that during the fermentation time, the carbohydrates of easy fermentation are converted into organic acids, like lactic and acetic acid, which reduce pH in the culture medium.
According to [38] the pH has an important effect in the microbial population because the growth and development of micro-organisms depends on pH in the environment. For present study, the highest pH values were observed in T1 and T2 at 0 and 24 h of fermentation. The alkalinity in the early fermentation stages besides of the low organic acids also may be related to the increase in total amino acids. These treatments also had the higher ammonia concentrations that also contributed to the increase in pH. In agreement, it has been found that pH values are closely related to NH3 production [4,39].
The concentration of LA increased with the increase in probiotic (PT) level at all fermentation times (P < 0.05); the higher concentrations were obtained in T3 and T4 at 48 and 72 h of fermentation. These changes in the lactic acid concentration may be associated with the growth of lactic acid bacteria during the fermentation time [40]. Similarly, [41] working on SSF with different levels of apple bagasse also reported increases in LA concen-

Table 2. Least square means of fermentative characteristics of sludge paper in SSF with inclusion of different levels of probiotic at different hours of fermentation.
tration. This sense, [42] studying different substrates and microorganisms in various combinations; they obtained an increase in LA concentration in SSF of crop residues with fungi and bacteria, mainly by strains of Rhizopus and Lactobacillus sp. Thus, the lactic acid is produced by the carbohydrates metabolism, and it can be used as indicator of fermentation in anaerobic conditions [8]. Usually, the production of LA tends to maintain the pH acid [43].
The N-NH3 decreased with the increase in PT level; but as the fermentation time advanced (P < 0.05), the N-NH3 concentration increased. The highest values of N-NH3 were found in T1 and T2 at 48 and 72 h of fermentation. These N-NH3 values could be related to the action of ureolytic enzymes of bacteria that normally occur in the SSF processes [22]. It is pointed out that urea in SSF is transformed by the action of microorganisms to N-NH3 [44]. This factor can also be produced by deamination of the non-protein nitrogen compounds [45].
The cells counts of yeasts per ml (Log 10) increased with the increase in PT level at all times of fermentation (P < 0.05). The highest values of yeast counts (YC) were obtained in T2 at 72 h, and in T3 and T4 at 48 and 72 h of fermentation. These results can be related with proper pH (acid) required for the growth of these organisms. The probiotic increased the yeast presence by the nature of this product which is rich in these microorganisms. In addition, the fermentable carbohydrates in SSF favored the development of certain microorganisms that are naturally present in substrates [18]. The results obtained in present study are in agreement with [46] who reported increases in yeast content using molasses as substrate and yeast Kluyveromices lactis obtained from apple (Malus domestica) in SSF process. Similarly, [47] found increases in microbial counts in SSF of feeds using mixed cultures of Lactobacillus fermentum, Saccharomyces cerevisiae and Bacillus subtilis; and they also obtained an increase in microbial protein. The improvement in nutritional quality of some fermented substrates can largely be attributed to the increase in the yeast population; this condition is supported by the increase in true protein.
4. CONCLUSION
According to the results of the present experiment, the best treatment was T4 (150 g/kg of probiotic) where the highest crude protein values and yeast counts were observed. The better percentages of true protein were obtained in T4 at 72 h of fermentation. It is feasible for the solid state fermentation of sludge paper with the use of probiotics, where the production of microbial protein is important in the animal feed. The increase in the nutriational quality of sludge paper treated with Prozoot15® can be attributed to the increase in microbial population.
REFERENCES
- Murrieta, A.J. (1996) Caracterización del valor nutritivo de subproductos de la elaboración de papel. Programa especial de investigación. Facultad de Zootecnia y Ecología, Universidad Autónoma de Chihuahua, Chihuahua.
- Díaz, A.I., Vilches, P. and Salas, O. (2000) Aprovechamiento de residuos, lodos papeleros para barras hidráulicas. Facultad de ingeniería Química, País Vasco.
- Mennickent, S. and Green, K. (2009) Los probióticos y su utilidad terapéutica. Ciencia Ahora, 24, 31-38.
- Elías, A., Lezcano, O. and Herrera, F.R. (2001) Algunos indicadores bromatológicos y productos finales de la fermentación de cuatro tipos de saccharina inoculados con vitafert. Revista Cubana de Ciencia Agrícola, 35, 153- 158.
- Cruz, G.D. (2008) Niveles de Inclusión de residuos de panadería en la fermentación en estado sólido del bagazo de manzana. Tesis de Licenciatura, Facultad de Zootecnia y Ecología, Universidad Autónoma de Chihuahua, Chihuahua.
- Shim, Y.H., Shinde, P.L., Choi, J.Y., Kim, J.S., Seo, D.K., Pak, J.I., Chae, B.J. and Kwon, I.K. (2010) Evaluation of multi-microbial probiotics produced by submerged liquid and solid substrate fermentation methods in broilers. AsianAustralasian Journal of Animal Science, 23, 521-529.
- Madrid, J., Martínez-Teruel, A., Hernández, F. and Megías, M.D. (1999) A comparative study on the determination of lactic acid in silage juice by colorimetric, high-performance liquid chromatography and enzymatic methods. Journal of the Science of Food and Agriculture, 79, 1722-1726. http://dx.doi.org/10.1002/(SICI)1097-0010(199909)79:12<1722::AID-JSFA427>3.0.CO;2-S
- Rodríguez, Z., Elías, A., Bocourt, R. and Nuñez, O. (2001) Efectos de los niveles de nitrógeno ureico en la síntesis proteica durante la fermentación de mezclas de caña (Saccharum officinarum) y boniato (Ipomea batata Lam.). Revista Cubana de Ciencia Agrícola, 35, 29-36.
- Broderick, G.A. and Kang, H.J. (1980) Automated simultaneous determination of ammonia and amino acids in ruminal fluid and in vitro media. Journal Dairy Science, 63, 64-75. http://dx.doi.org/10.3168/jds.S0022-0302(80)82888-8
- A.O.A.C. (1995) Official method of analysis. 16th Edition, Association of Official Analytical Chemists, Washington DC.
- ANKOM (2005) Methods for determining acid detergent fiber. Automated fiber analysis. ANKOM technology. http://www.ankom.com/
- ANKOM (2005) Methods for determining neutral detergent fiber. Automated fiber analysis. ANKOM technology. http://www.ankom.com/
- SAS (2002) SAS/Stat user’s guide. SAS Institute Inc., Cary.
- Ruíz, C.J., Ruíz, M., Ruíz, G. and Torres, V. (2002) Effect of Inclusion of ammonium sulfate on the elaboration of rustic saccharina. Cuban Journal of Agricultural Science, 36, 147-152.
- Chiang, G., Lu, W.Q., Piao, X.S., Hu, J.K., Gong, L.M. and Thacker, P.A. (2010) Effects of feeding solid-state fermented rapeseed meal on performance, nutrient digestibility, intestinal ecology and intestinal morphology of broiler chickens. Asian-Australasian Journal of Animal Science, 23, 263-271.
- Carrasco, E., Elías, A., Martin, P., Valiño, E. and Febles, I. (1997) Tiempo de fermentación de la caña de azúcar con excreta vacuna. Revista Cubana de Ciencia Agrícola, 31, 45-48.
- Elías, A., Lezcano, O., Lezcano, P., Cordero, J. and Quintana, L. (1990) Reseña descriptiva sobre el desarrollo de una tecnología de enriquecimiento proteico de la caña de azúcar mediante fermentación en estado sólido (Saccharina). Revista Cubana Ciencia de Agrícola, 24, 3-12.
- Rodríguez, Z., Bocourt, R., Elías, A. and Madera, M. (2001) Dinámica de fermentación de mezclas de caña (Saccharum officinarum) y boniato (Ipomea batata). Revista Cubana de Ciencia Agrícola, 35, 147-151.
- FEDNA (2003) Subproductos de cereales. Tablas FEDNA de composición y valor nutritivo de alimentos para la fabricación de piensos compuestos. Segunda Edición, Fundación Española para el Desarrollo de la Nutrición Animal. http://www.etsia.upm.es/fedna/mainpageok.htm.
- Pérez, Q.L.M. (1996) Fermentación en estado sólido del mijo perla (Pennisetum americanum) por Rhizopus oligosporus para la obtención de un producto rico en proteína. Tesis de Maestría, Facultad de Ciencias Biológicas, Universidad Autónoma de Nuevo León, Monterrey.
- Acuña, V.S. (2004) Producción de aceite y grasa por fermentación. http://www.revistaciencias.com/publicaciones/EpZZEuZkFkAWIRtUkS.p.p/
- Valiño, E., Elías, A., Torres, V. and Albelo, N. (2002) Study of the microbial content fresh sugarcane bagasse as substrate for animal feeding by solid state fermentation. Cuban Journal of Agricultural Science, 36, 359-364.
- Márquez, A.T., Mendoza, G.D., González, S.S., Buntinx, S.E. and Loera, O. (2007) Actividad fibrolitica de enzimas producidas por Tramete ssp. EUM1, Pleurotusostreatus IE8 y Aspergillus niger AD96.4 en fermentación solida (Reporte). Interciencia. http://www.highbeam.com .
- Fazaeli, H., Aziz, A., Jelan, Z.A. and Mirhadi, S.A. (2003) Effect of fungal treatment on the chemical composition, in vitro digestibility and in sacco degradability of wheat straw. Proceedings British Society of Animal Science, 6, 845-852.
- Sun, X., Zhang, R. and Zhang, Y. (2004) Production of lignocellulolytic enzymes by Trametes gallica and detection of polysaccharide hydrolase and lactase activities in polyacrylamida gels. Journal of Basic Microbiology, 44, 220-231. http://dx.doi.org/10.1002/jobm.200310376
- Ibarra, A., García, Y., Valiño, E., Dustet, J., Albelo, N. and Carrasco, T. (2002) Influence of aeration on the bioconversion of sugarcane bagasse by Trichoderma viride M5-2 in a static bioreactor of solid fermentation. Cuban Journal of Agricultural Science, 36, 152-158.
- Joshi, V.K. and Sandhu, D.K. (1996) Preparation and evaluation of an animal feed byproduct produced by solid-state fermentation of apple pomace. Bioresource Technology, 56, 251-255. http://dx.doi.org/10.1016/0960-8524(96)00040-5
- Ortiz, G., Miranda, J.L., Cerrillo, M.A., Reyes, A.J., Torres, E.F. and Cruz, O.S. (2007) Effect of solid substrate fermentation on the nutritional quality of agro-industrial residues. Intercencia, 32, 339-343.
- Villas-Boas, S.G., Esposito, E. and De Mendonca, M.M. (2003) Bioconversion of apple pomace into a nutritionally enriched substrate by Candida utilis and Pleurotus ostreatus. World Journal of Microbiology and Biotechnology, 19, 461-467. http://dx.doi.org/10.1023/A:1025105506004
- Ramos, J., Elías, A. and Herrera, F. (2005) Efecto de cuatro fuentes energéticas en la fermentación en estado sólido (FES) de la caña de azúcar. Memorias del I Congreso Internacional de Producción Animal, La Habana, 298- 302.
- Pandey, A., Soccol, C.R., Rodríguez-León, J.A. and Nigam, P. (2001) Solid-state fermentation in Biotechnology. Fundamentals and Applications. Asiatech Publishers, Inc., New Delhi.
- Berradre, M., Mejías, M., Ferrer, J., Chandler, C., Páez, G., Mármol, Z., Ramones, E. and Fernández, V. (2009) Fermentación en estado sólido del desecho generado en la industria vinícola. Revista Facultad de Agronomía, 26, 398-422.
- Becerra, A., Rodríguez, C., Jiménez, J., Ruiz, O., Elías, A. and Ramírez, A. (2008) Urea y maíz en la fermentación aeróbica de bagazo de manzana para la producción de proteína. Tecnociencia Chihuahua, 2, 7-14.
- Van-Winsen, R.L., Urlings, B.A.P., Lipman, L.J.A., Snijders, J.M.A., Keuzenkamp, D., Verheijden, J.H.M. and VanKnapen, F. (2001) Effect of fermented feed on the microbial population of the gastrointestinal tracts of pigs. Applied and Environmental Microbiology, 67, 3071-3076. http://dx.doi.org/10.1128/AEM.67.7.3071-3076.2001
- Canibe, N., Virtane, E. and Jensen, B.B. (2006) Microbial and nutritional characteristics of pig liquid feed during fermentation. Animal Feed Science and Technology, 134, 108-123. http://dx.doi.org/10.1016/j.anifeedsci.2006.05.005
- Canibe, N., Miettinen, H. and Jensen, B.B. (2008) Effect of adding Lactobacillus plantarum or a formic acid containing product to fermented liquid feed on gastrointestinal ecology and growth performance of piglets. Livestock Science, 144, 251-262. http://dx.doi.org/10.1016/j.livsci.2007.05.002
- Morgan, F. (2003) La pulpa de café enriquecida. Un aporte al desarrollo sostenible en la zona montañosa de Guantánamo. Ph.D. Tesis, Instituto de Ciencia Animal, La Habana.
- Bertran, E., Sort, X., Soliva, M. and Trillas, I. (2004) Composting winery waste: Sludges and grapes stalks. Bioresource Technology, 95, 203-208. http://dx.doi.org/10.1016/j.biortech.2003.07.012
- Rodríguez, Z. (2005) Uso del boniato (Ipomea batata. Lam) en la fermentación en estado sólido de la caña de azúcar (Saccharum officinarum). Ph.D. Tesis, Instituto de Ciencia Animal, La Habana.
- Castillo, Y. (2009) Fermentación in vitro para obtener la levadura Candida norvogensis en mezclas de alfalfa con bagazo de manzana fermentado y sus efectos sobre la actividad microbiana ruminal. Ph.D. Tesis, Facultad de Zootecnia y Ecología, Universidad Autónoma de Chihuahua.
- Angulo, C. (2008) Niveles de inclusión de bagazo de manzana en fermentación en estado sólido en lodos de papel. Programa Especial de Investigación, Facultad de Zootecnia y Ecología, Universidad Autónoma de Chihuahua.
- Chundakkadu, K. (2005) Solid state fermentation systems—An overview. Critical Reviews Biotechnology, 25, 1-30. http://dx.doi.org/10.1080/07388550590925383
- Flores, N.M. (2000) Elaboración de cultivos microbianos a partir de pasta de coco y su utilización en dietas para borregos en engorda. Tesis de Maestría, Universidad de Colima, Colima.
- Rodríguez, H.E. (2009) Producción y evaluación de alimentos fermentados a partir de bagazo y desecho de manzana y su efecto sobre el desarrollo ruminal y pará- metros sanguíneos. Ph.D. Tesis, Facultad de Zootecnia y Ecología, Universidad Autónoma de Chihuahua, Chihuahua.
- Calderón, J.O., Elías, A. and Valdivie, M. (2005) Diná- mica de la fermentación en estado sólido de las camas de cascarilla de café en inicio de ponedoras inoculadas con vitafert. Revista Electrónica de Veterinaria, (REDVET), VI. http://www.veterinaria.org/revistas/redvet/n050505/050521.pdf
- Díaz, D., Rodríguez, C., Mancillas, P., Angulo, C., Salvador, F., Ruiz, O., Rubio, H.O., Mena, S. and Elías, A. (2011) Desarrollo de un inóculo con diferentes sustratos mediante fermentación sólida sumergida. Revista Electrónica de Veterinaria (REDVET), 12, 1-10.
- Hu, J., Lu, W., Wang, C., Zhu, R. and Qiao, J. (2008) Characteristics of solid-state fermented feed and its effects on performance and nutrient digestibility in growing-finishing pigs. Asian-Australasian Journal of Animal Science, 21, 1635-1641.
LIST OF ABBREVIATIONS
ADF: Acid Detergent Fiber
CP: Crude Protein
DM: Dry Matter
g: gram
h: hour
kg: kilogram
LA: Lactic Acid
N-NH3: Ammonia Nitrogen
NDF: Neutral Detergent Fiber
pH: Concentration of hydrogen ions
PT: Probiotic
PS: Paper Sludge
SSF: Solid State Fermentation
T: Treatments
TP: True Protein
YC: Yeast Counts
NOTES
*Corresponding author.

