Journal of Materials Science and Chemical Engineering
Vol.04 No.02(2016), Article ID:64017,6 pages
10.4236/msce.2016.42014
Determination of Total Flavonoids and Its Antioxidant Ability in Houttuynia cordata
Anyin Chen1,2*, Wenjun Xiang1,2, Dan Liu1, Changlu Liu1, Li Yang1
1Key Laboratory of Exploitation and Study of Distinctive Plants in Education Department of Sichuan Province, Sichuan University of Arts and Science, Dazhou, China
2Department of Chemistry and Chemical Engineering, Sichuan University of Arts and Science, Dazhou, China

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 8 December 2015; accepted 25 February 2016; published 29 February 2016
ABSTRACT
The total flavonoids of Houttuynia cordata are determined by UV-Vis spectrophotometry. The extraction condition of flavonoids from Houttuynia cordata includes: the extraction temperature is 70˚C, solid-liquid ratio is 1:20, solvent is 80% ethanol, extraction time is 65 min. We studied the antioxidation of flavonoids in Houttuynia cordata, and results showed that flavonoids had strong reducing capacity, hydroxyl free radical scavenging activity and the superoxide anion radical scavenging ability, thus can provide a basis for the development of natural antioxidants.
Keywords:
Houttuynia cordata, Total Flavonoids, Antioxidant Capacity

1. Introduction
As a common plant, Houttuynia cordata is widely distributed in the area of southeast, central region and southwest of China. It is not only used as great food material, but also a medicine for curing various diseases. Containing flavonoids anti-cancer agent, the Houttuynia cordata has preferable effects on the treatment of gastric cancer and certain therapeutical effects on lung cancer and colorectal cancer of the middle and advanced stage [1] . Apart from that, it also has a function with healthcare, such as commonly used in the healthcare food like tea drinks.
Since flavonoids could not be synthesized in the body, it is merely brought from plants. Therefore, to better extract and separate flavonoids from the Houttuynia cordata, the determination of the total amount of flavonoids and in vitro antioxidant capacity are carried out, providing references for the reasonable utility of the resource, as well as the theoretical basis and technical support for quality control and pharmacological activity research.
2. Experimental Part
2.1. Materials and Instruments
Ultraviolet and visible spectrophotometer (UV-2550), centrifugal machine (TG20-WS), electronic scales (FA1104N), draught drying cabinet (101-2AB), herbal medicine grinding machines (FW-135), sieve (60 mesh). Houttuynia cordata (bought from Dazhou, Sichuan) was proceeded with wash, natural withering, drying to constant weight, and then smash for use. The reagents include rutin standard sample, ethyl alcohol, ethyl alcohol, aluminium nitrate, sodium nitrite, distilled water, petroleum ether, trichloroacetic acid and vitamin C.
2.2. Experiment Method
2.2.1. Standard Curve of Rutin
Twenty milligram of rutin standard sample is precisely weighed and dissolved in the solution with 40% ethyl alcohol. Then, the solution was transferred to the volumetric flask of 100 mL prior to metered volume and shaking up. In this way, a solution with mass concentration of 0.2 mg rutin per milliliter is obtained. A series amount of the standard solution, including 0, 1.0 ml, 2.0 ml, 1.0 ml, 6.0 ml, 8.0 ml and 10.0 ml, are added into seven 25 ml volumetric flasks, respectively. Then 12.5 mL 40% ethanol solution, 0.5 mL 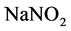 (1:20) solution with mass fraction of 5%, are added in each of the volumetric flasks, and left for 5 min. The
(1:20) solution with mass fraction of 5%, are added in each of the volumetric flasks, and left for 5 min. The 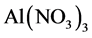 solution with the mass fraction of 10% was injected prior to shaking up. After left for 5 min, 4 mL sodium hydroxide solution (1:25) was added, and the solution was homogeneously mixed. Finally, 40%
solution with the mass fraction of 10% was injected prior to shaking up. After left for 5 min, 4 mL sodium hydroxide solution (1:25) was added, and the solution was homogeneously mixed. Finally, 40% 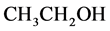 was added to calibrate the mixture, and then left for 10 min. With distilled water as blank control, the absorbance of the solution was measured at 510 nm by ultraviolet spectrophotometer. The Rutin standard curve was drawn with the rutin concentration as abscissa and absorbance the ordinate [2] .
was added to calibrate the mixture, and then left for 10 min. With distilled water as blank control, the absorbance of the solution was measured at 510 nm by ultraviolet spectrophotometer. The Rutin standard curve was drawn with the rutin concentration as abscissa and absorbance the ordinate [2] .
2.2.2. The Extraction Condition of Flavonoids
The grass of Houttuynia cordata was dried, smashed and sieved to 60 mesh, prior to immersing into petroleum ether for 2 h (Figure 1). After the supernatant was discarded, petroleum ether was added to immerse the grass again, until the colorless supernatant was observed. Then the sample was dried with drying cabinet (70˚C). Four grams of the Houttuynia cordata powder was taken into return pipe of the Soxhlet extractor along with 120 ml 80%
Figure 1. Standard curve of rutin.
ethanol solvent. Reflux extraction was carried out at 70˚C for 65 min [3] . Flavonoids filtrate was obtained by vacuum filtration from the extract. After five milliliter of flavonoids filtrate was fetched from the volumetric flask, a solution containing 5 percent of 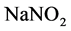 was added into the mixture before homogenously mixed and left for 5 min. Similarly, the solution of 10 percent
was added into the mixture before homogenously mixed and left for 5 min. Similarly, the solution of 10 percent 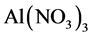 was added, mixed and left for 1.5 min, before 4 mL 1 mol/L sodium hydroxide was mixed. In the end, 40% ethyl alcohol was used to calibrate, then it was left for 10 min. The flavonoids content was calculated by the method of determining absorbance using ultraviolet spectrophotometer.
was added, mixed and left for 1.5 min, before 4 mL 1 mol/L sodium hydroxide was mixed. In the end, 40% ethyl alcohol was used to calibrate, then it was left for 10 min. The flavonoids content was calculated by the method of determining absorbance using ultraviolet spectrophotometer.
2.2.3. The Calculation of Flavonoids Content
According to the experimental process, the amount of extract could be indicated as follows:
The content of flavonoids: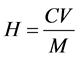 .
.
C is the concentration of the extract, mg/ mL; V is the volume of the extract, mL; M is the mass of the sample, g.
The absorbance of the Houttuynia cordata sample was tested to be 0.574, and the responding concentration was 0.048 mg/mL according to the standard curve. Therefore, the content of the flavonoids was 12 mg/g.
2.2.4. Research on the Reducing Power of Flavonoids
The filtrate was concentrated into a flavonoids-ethanol solution with mass concentration of 500 mg/L, in order to examine the antioxidant activity.
By using the prepared solution, a series of flavonoids sample solution were obtained, of which the concentration were 10 mg/L, 20 mg/L, 30 mg/L, 40 mg/L and 50 mg/L, respectively. Taking 1.0 ml different mass concentration of flavonoids sample solution, 3.0 mL 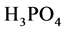 (pH = 6.6, 0.2 mol/L) and 2.5 ml solution which contains 1% mass fraction of Fe (SCN) 3 into a 10 ml centrifuge tube, the mixture was homogenously mixed and maintained at 60˚C for 25 min. Afterwards, the mixture was subject to cold water and amended with 2.5 mL 10% trichloroacetic acid, and the distilled water was used for calibration. After the centrifugation in a speed of 2500 r/min, 2.5 mL supernatant was taken to mix 0.1% 1.0 mL
(pH = 6.6, 0.2 mol/L) and 2.5 ml solution which contains 1% mass fraction of Fe (SCN) 3 into a 10 ml centrifuge tube, the mixture was homogenously mixed and maintained at 60˚C for 25 min. Afterwards, the mixture was subject to cold water and amended with 2.5 mL 10% trichloroacetic acid, and the distilled water was used for calibration. After the centrifugation in a speed of 2500 r/min, 2.5 mL supernatant was taken to mix 0.1% 1.0 mL 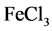 and distilled water. 15 min later, its absorbance was determined at 700 nm using ultraviolet spectrophotometer. Distilled water and vitamin C were used as reference and control, respectively.
and distilled water. 15 min later, its absorbance was determined at 700 nm using ultraviolet spectrophotometer. Distilled water and vitamin C were used as reference and control, respectively.
2.2.5. Research on the Hydroxyl Free Radical Scavenging Ability of Flavonoids
By using certain amount of the standby solution, 10 ml flavonoids sample solution were prepared under different concentration (50 mg/L, 100 mg/L, 150 mg/L, 200 mg/L, 250 mg/L). For flavonoids samples of different mass concentration, 1.0 ml of the solution prepared above was injected into the five test tubes, respectively. Then 1.0 ml/L 10 mmol/L FeSO4 and 1.0 ml 10 mmol/L salicylic acid-ethanol solution were added into the tube, which was consequently placed in a water bath at 37˚C for 35 min prior to taking out and calibration to the scale line with distilled water. Using ultraviolet spectrophotometer, the absorbance was measured at 510 nm with distilled water as a reference and vitamin C as control. The hydroxyl radical scavenging rate was calculated as follows [4] .
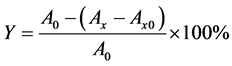
In the equation, Y represents the scavenging rate, A0 denotes the absorbance of control, Ax is the absorbance of the sample after the flavonoid solution was added, and Ax0 is the absorbance of flavonoids solution with no H2O2.
2.2.6. Research on the Superoxide Anion Radical Scavenging Ability of Flavonoids
By using certain amount of the standby solution, 10 ml flavonoids sample solution were prepared under different concentration (50 mg/L, 100 mg/L, 150 mg/L, 200 mg/L, 250 mg/L). For flavonoids samples of different mass concentration, 1.0 ml of the solution prepared above was injected into the five test tubes, respectively. Then 4.0 ml/L Tris (hydroxymethyl) metyl aminomethane- HCl buffer solution (pH = 8.2, 50 mmol/L) were added into the tube, which was consequently placed in a water bath at 25˚C for 25 min prior to the addition of 0.1 mL 25 mmol/L pyrogallic acid. After the mixing, the water bath was carried out again at 25˚C for 6 min. Then two droplets of 10 mol/L HCl solution were injected to terminate the reaction. Using ultraviolet spectrophotometer, the absorbance was measured at 325 nm with Tris (hydroxymethyl) metyl aminomethane-HCl buffer solution as a reference and vitamin C as control. The hydroxyl radical scavenging rate was calculated as follows [4] .
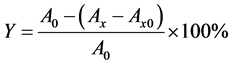
In the equation, Y represents the scavenging rate, A0 denotes the absorbance of control, Ax is the absorbance of the sample after the flavonoid solution was added, and Ax0 is the absorbance of flavonoids solution with no pyrogallic acid.
3 Results and Discussion
3.1. The Reducing Power of Flavonoids
The reduction ability mainly refers to determining whether the substance could provide electron for reducing oxidant. The extent of reduction ability was examined by comparing the absorbance value. The greater value of absorbance represents the stronger reduction ability. The reduction ability of substance has a close association with its antioxidant capacity, and a stronger material reducing ability indicates stronger oxidation resistance.
Figure 2 shows that in the tested concentration range, with the increase in the concentration of flavonoids in Houttuynia cordata. Furthermore, the reduction ability promoted by the flavonoids, appeared to be equivalent to vitamin C with the same mass concentration, indicating a preferable reduction ability of the flavonoids.
3.2. Elimination Ratio of Hydroxyl Radicals of Flavonoids
Hydroxyl free radicals, which is a kind of active oxygen radicals, is apt to attack other molecules, such as biological molecules, organic matter or inorganic matter. Hydroxyl free radicals work through a variety of interactions, including electron transfer, additive reaction and dehydrogenation, resulting in the oxidation of DNA, amino acids, protein, carbohydrate substances. Consequently, the cell necrosis or mutations emerges in the living organism.
In this experiment, the hydroxyl radicals can react with salicylic acid and generate colored product which has strong absorption at the wavelength of 530 nm. The addition of a substance that possesses hydroxyl radical scavenging ability to compete with salicylic acid will lower the yield of the colored product resulting from hydroxyl radical oxidation.
Figure 2. The reducing power of flavonoids in Houttuynia cordata.
As is seen from Figure 3, in the tested mass concentration range, hydroxyl free radical scavenging effect of the flavonoids is enhanced by the increase in the concentration of flavonoids. As a result, flavonoids have stronger hydroxyl radical scavenging ability than vitamin C.
3.3. Elimination Ratio of Superoxide Anion Radical of Flavonoids
The excessive superoxide anion free radical, due to its relative high activity, can cause damage to tissue of human body. The product resulted from the combination of superoxide anion free radical with hydroxyl radical in vivo, will finally lead to DNA damage in cell and normal function in human body. Under the alkaline condition, the oxidation will occur to the pyrogallic acid itself, and produces colored intermediate and superoxide anion, of which higher concentration result in faster oxidation rate of catalytic reaction. However, quick reaction of antioxidants with superoxide anion can inhibit the oxidation of pyrogallic acid.
Figure 4 shows that in the tested concentration range, the flavonoids have the ability to remove superoxide
Figure 3. Elimination ratio of hydroxyl radicals of flavonoids.
Figure 4. Elimination ratio of superoxide anion radical of flavonoids.
anion free radicals. Higher mass concentration of Houttuynia cordata flavonoids give rise to the greater scavenging effect of superoxide anion free radical, which is equivalent to that of the vitamin C, in the range of mass concentration from 0 to 50 mg/L. When the mass concentration is greater than 50 mg/L, scavenging effect of superoxide anion free radical appeared to be relative low, compared to vitamin C, and the difference increases with the increase of the mass concentration.
In northeast area of Sichuan, total content of flavonoids in the whole grass of Houttuynia cordata powder sample is 12 mg/g.
4. Conclusions
The result of antioxidant test in vitro indicates that flavonoids in Houttuynia cordata have strong reducing ability, and their ability of reducing is comparable to vitamin C with similar mass concentration. Compared with the vitamin C in the same mass concentration, flavonoids in Houttuynia cordata possess weak superoxide anion radical scavenging ability; in contrast, the hydroxyl radical scavenging ability of flavonoids is stronger.
In summary, flavonoid is not only a kind of good natural antioxidant, but also a natural health product. Therefore, it will be widely used as a functional food and medicine in the future. Since many problems exist in the synthesized antioxidants, such as insecurity and deleteriousness, the natural antioxidant will gain a larger market advantage and will be better accepted by us.
Acknowledgements
This study was financially supported by the scientific research fund of Key Laboratory of Exploitation and Study of Distinctive Plants in Education Department of Sichuan Province (sctz201306).
Cite this paper
AnyinChen,WenjunXiang,DanLiu,ChangluLiu,LiYang, (2016) Determination of Total Flavonoids and Its Antioxidant Ability in Houttuynia cordata . Journal of Materials Science and Chemical Engineering,04,131-136. doi: 10.4236/msce.2016.42014
References
- 1. Abdolghaffari, A.H., Baghaei, A., Moayer, F., Esmaily, H., Baeeri, M., Monsef-Esfahani, H.R., et al. (2010) On the Benefit of Teucrium in Murine Colitis through Improvement of Toxic Inflammatory Mediators. Human & Experimental Toxicology, 29, 287-295.
http://dx.doi.org/10.1177/0960327110361754 - 2. Sharififar, F., Dehghn-Nudeh, G. and Mirtajaldini, M. (2009) Major Flavonoids with Antioxidant Activity from Teucrium polium L. Food Chemistry, 112, 885-888.
http://dx.doi.org/10.1016/j.foodchem.2008.06.064 - 3. Hinneburg, I., Dorman, H.J.D. and Hiltunen, R. (2006) Antioxidant Activities of Extracts from Selected Culinary Herbs and Spices. Food Chemistry, 97, 122-129.
http://dx.doi.org/10.1016/j.foodchem.2005.03.028 - 4. Ghafar, M.F.A., Prasad, K.N., Weng, K.K. and Ismail, A. (2010) Flavonoid, Hesperidine, Total Phenolic Contents and Antioxidant Activities from Citrus Species. African Journal of Biotechnology, 9, 326-330.
NOTES

*Corresponding author.





