Agricultural Sciences
Vol. 3 No. 7 (2012) , Article ID: 24662 , 10 pages DOI:10.4236/as.2012.37117
Use of carnauba based carrier for copper sprays reduces infection by Xanthomonas citri subsp. citri and Diaporthe citri in Florida commercial grapefruit groves
![]()
1USDA/ARS, US Horticultural Research Laboratory, Fort Pierce, USA; *Corresponding Author: jan.narciso@ars.usda.gov
2IFAS, IRRC, University of Florida, Fort Pierce, USA
3PACE International, Wenatchee, USA
Received 18 August 2012; revised 21 September 2012; accepted 3 October 2012
Keywords: Citrus Canker; Melanose; Protective Sprays; Adjuvant; Coatings
ABSTRACT
Citrus canker, caused by Xanthomonas citri subsp. citri (Xcc), is a bacterial disease of citrus and results in peel blemishes rendering fresh fruit unsalable. Xcc is most active in warm, wet Florida summers where tissues are infected during periods of active growth. Melanose, caused by Diaporthe citri, is common in citrus producing countries, but, like canker, is only important for fresh market fruit. To control canker and melanose, Florida growers spray trees with copper formulations (Cu), but these sprays are removed by strong rains and intense radiation of Florida summers. A study was undertaken in FL commercial grapefruit groves in 2009 and 2010 to assess the efficiency of a spray combining copper with a specially formulated, hydrating wax (WashGard®) (WG). Using a 21-day spray schedule for the season, fruit were sprayed with WG + Cu, Cu and Control (no spray). Fruit from trees sprayed with WG + Cu had approximately 10 and17% more canker free fruit in 2009 and 2010 respectively compared to trees sprayed with copper alone. Compared to control trees the canker free fruit incidence was increased by ≈10% in 2009 and 57% in 2010. For melanose there was 40% more disease free fruit (treated) over fruit from trees with no treatment in 2009 and approximately 20% more in 2010. Controlling infection with this spray significantly reduces citrus canker and melanose, increasing the percentage of marketable fruit.
1. INTRODUCTION
Citrus canker, caused by Xanthomonas citri subsp. citri (Xcc) is found on all citrus cultivars, with grapefruit, Mexican lime and lemon being the most sensitive [1-4] (Figure 1(a)). All parts of the plant are susceptible but young tissue is the most vulnerable [1,2]. In warm, wet, weather the bacteria ooze from the lesions and are carried in wind driven rain to susceptible tissue. On fruit, canker affects the peel producing superficial lesions, which render the fruit unmarketable for fresh sales. One of the strongest impacts of canker is that it causes severe restrictions on fruit movement to some markets [4].
Melanose (Diaporthe citri F. A. Wolf; anamorph Phomopsis citri H. Fawc. Non (Sacc.) Traverso & Spessa) is a fungal disease of citrus that causes a superficial blemish on the peel reducing acceptability in the fresh market [5] (Figure 1(b)). All citrus varieties grown in Florida are susceptible but as in canker, grapefruit are the most sensitive [5-7]. Disease severity is regulated by temperature and rainfall conditions during leaf expansion [6-9]. In humid and wet weather, conidia exude from pycnidia in dead wood and are washed down the tree from the canopy [5,6,8,9].
Copper formulations (e.g. copper hydroxide) are routinely sprayed on citrus in Florida on a 3-week (21 day) schedule for control of canker, melanose and other fungal diseases [6]. MCGuire [3] found that copper containing sprays were effective in reducing epiphytic populations of Xcc. However, copper dries to a powder and is easily washed away with rains; it also erodes in the harsh UV light and dry conditions in Florida groves. Since the bacterial and fungal pathogens are most problematic during rain and wind events in the summer, the dissolution of copper under these conditions leads to unprotected susceptible tissue. It is critically important that successful protective sprays are available to reduce infection as well
 (a)
(a) (b)
(b)
Figure 1. (a) Citrus canker on leaves, immature and mature fruit; (b) Melanose on leaves, immature and mature fruit.
as inoculum in the groves.
It is well documented that there are many factors causing erosion and deterioration of fungicides/bactericides on plant tissues [10-13]. Rainfall has one of the most important effects on the persistence of field sprays [14-17]. In greenhouse studies, fungicides (including copper hydroxide) applied before inoculation with Diaporthe, offered only 50% control for 2 days with simulated rain [18]. These same fungicides gave little postinoculum protection [18,19]. Plant surface topography, attraction of spray particles for the sprayed surface, elasticity of sprays during tissue expansion, and size of spray droplets are just a few of the issues involved in consideration of effectiveness of protective sprays [10-15,20].
For many years, plant pathologists have used sticking agents (e.g. oils] in an effort to keep spray compounds on plant tissues [21,22]. Recent studies with anti-transpirant polymer coatings have shown that some of these compounds can reduce disease by forming both a mechanical barrier between pathogens and plant tissue as well as changing the chemical characteristics of plant tissues [9, 10,23]. Little is known about the commercial use of these coatings for disease control [9]. Various adjuvants have been mixed with sprays to protect Florida commodities. However, none have made a significant difference in the persistence of these treatments and both citrus canker and melanose remain a problem for Florida fresh market fruit.
In this study, a formulation of carnauba wax was used to aid sticking of copper hydroxide to citrus trees during the Florida summer rainy season. It also helped maintain moisture within the coating to keep the copper active for a longer period. The copper/wax formulation was applied in commercial grapefruit groves during the 2009, 2010 growing seasons to test efficiency.
2. MATERIALS AND METHODS
2.1. Sprays
Commercial groves in central and south Florida were used for these experiments. Studies on canker and melanose control were undertaken concurrently with the same fruit assessed for both melanose and canker post harvest. In 2009 the groves had high disease pressure for both canker and melanose. The experimental plot in Grove A consisted of 20 adult heavy bearing trees (>10 years old), except where diseased trees had been culled and young trees planted. Trees with different treatments were mixed in 2 rows (10 trees in each row) with several untreated rows between. Rows were unprotected on the west and southwest sides of the plot due to culled diseased trees. Experimental trees were randomly treated with 2 experimental treatments consisting of: (WG + Cu)- carnauba formulation (WashGard®, Pace International, Wenatchee, WA) mixed with copper hydroxide (Kocide 3000, Dupont, NJ), (Cu)-copper hydroxide only, along with a (control) with no treatment. Both spray mixtures were formulated to contain 1.5 lbs metallic copper equivalent per acre and the spray with WashGard® also contained 2.5 gallons WG per 100 gallons spray. The rate of copper used corresponded to that commonly used by commercial growers for canker control. Preliminary experiments spraying with WG alone showed no protective effect and were not continued. Sprays were applied every 3 weeks from fruit size ≈3 cm (1.2 in) to maturation (April through October) using a Stihl backpack sprayer (Stihl SR 420, Virginia Beach, VA) with a 13 L capacity. Sprays were mixed on site and sprayed until run off. Plastic covered, dark paper was hung in the middle of each tree to insure that the interiors of the trees were sprayed.
During 2010 tests were expanded to 3 grove areas. Situated in the same grove that was used in 2009 (Grove A), a row of 81 young trees (<5 years) was utilized. All trees in this part of the grove were exposed to wind. Three trees at each end of the row were used as buffers and not included. The remaining 75 trees were divided into 15 plots, 3 trees per plot with 2 untreated trees between each plot. Experimental treatments were randomly assigned to each plot for 3 test spray treatments: (WG + Cu/Wk3)-WashGard® with copper hydroxide sprayed every 3 weeks, (Cu/Wk3)-copper hydroxide alone sprayed every 3 weeks, and (WG + Cu/6Wk)-WG + Cu sprayed every 6 weeks to further test spray persistence. The 30 trees between plots that were not sprayed were used as controls.
Grove area B was a commercial grove with experimental plots areas totaling 24 adult trees interspersed with 24 control trees. Grove B was sprayed only with WG + Cu every 3 weeks as an experimental spray and compared with unsprayed control trees. Grove area C was set up as in Grove B, but with 18 trees total sprayed with WG + Cu every 3 weeks and compared to 24 unsprayed control trees. All spray mixtures in 2010 were formulated and sprayed to coat each tree as done in 2009. In 2010, disease pressure for canker was extremely high in all areas while melanose disease pressure was moderate in all areas. All grove areas in 2010 had been sprayed with WG the previous year.
2.2. Harvest
In both years, final sprays were done in October with harvest approximately 5 weeks later. In 2009, eight thousand five hundred total grapefruit were harvested with only a slight variation in numbers between treatments. In 2010, about 2000 total fruit were harvested as the trees were younger with fewer fruit. Fruit from each grove were separated by treatment, placed in plastic crates, transported to the Citrus and Subtropical Products USDA/ARS lab in Winter Haven and stored at 18˚C (64˚F). Canker and melanose infection were then evaluated using disease assessment keys.
2.3. Statistical Analysis
Analysis was run on data using NCSS Statistical Software (Kayesville, UT) and Categorical Data Analysis (Fisher’s Exact Test) from Statistical Research Methods in the Life Sciences [24,25].
2.4. Results
Results for both 2009 and 2010 were determined using disease assessment keys, a standard method for assessing disease in the field and on field commodities [26-28]. For both years and in all grove areas data show that trees sprayed with WG + Cu had significantly more unblemished (canker free) fruit than both the untreated controls and trees sprayed with copper alone. The increase in WG + Cu unblemished fruit in 2010 when compared with 2009 suggests a cumulative protective effect when sprayed consistently.
For 2009 in grove A, fruit treated with WG + Cu had almost 10% more blemish free fruit than those treated with the copper alone (P < 0.003) or untreated controls (P < 0.001) (Figure 2). There was no difference found between untreated fruit and fruit treated with copper alone.
When assessed for melanose, fruit from trees with the WG + Cu treatments had significantly more blemish free fruit than from trees sprayed only with copper (P < 0.01) or the untreated controls (P < 0.01). The trees treated with WG + Cu had almost 40% unblemished fruit, those treated with copper alone had only 23% unblemished fruit, and the untreated the control had less than 10% unblemished fruit (Figure 3). Spray treatments of WG without copper were not significantly different from the control (data not shown).
In 2010, two additional areas in different groves (B and C) were treated and compared with young trees from the original grove. While the young trees in grove A were evaluated for differences between WG + Cu and copper alone sprayed every 3 weeks, controls (no treatment) and

Figure 2. Percent of canker free (unblemished) grapefruit harvested in 2009 from grove A after treatments of WashGard® + Copper (WG + Cu) or Copper only (Cu) sprays applied every 3 weeks compared to unsprayed control.
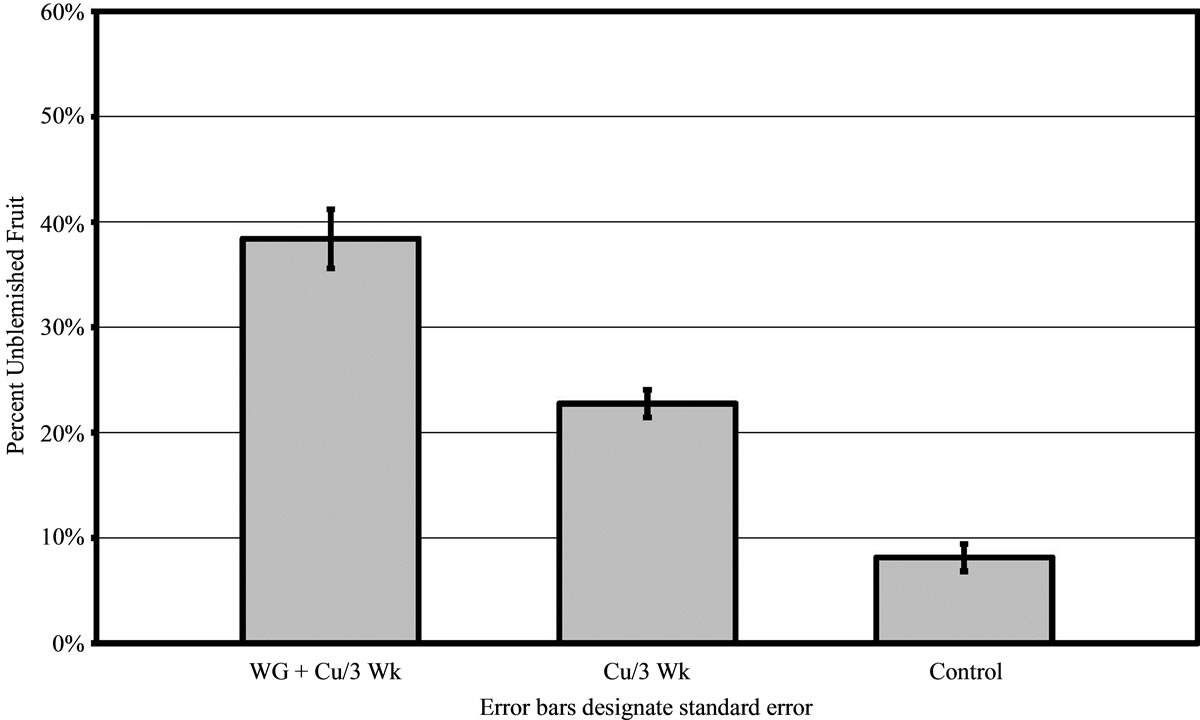
Figure 3. Percent of melanose free (unblemished) grapefruit harvested in 2009 from grove A after treatments of WashGard® + Copper (WG+Cu) or Copper only (Cu) sprays applied every 3 weeks compared to unsprayed control.
WG + Cu sprayed every 6 weeks instead of the suggested 3-week spray schedule; treatments in groves B and C were only tested with WG + Cu sprayed every 3 weeks and compared to control trees with no treatment since the treatments with Cu alone were not different from the no spray control in 2009. The 6-week interval spray trial was established to see if the activity of WG + Cu would allow growers to spray less often and obtain results equivalent to the standard spray schedule.
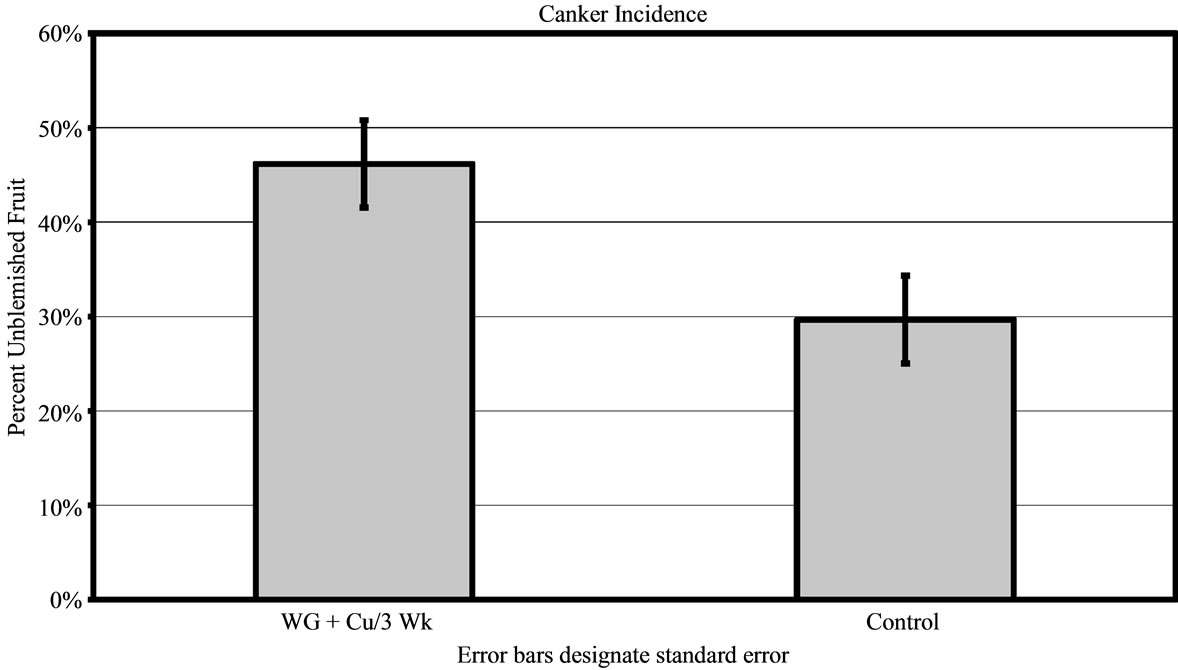
For 2010, results were analogous to 2009 in all areas. In grove A, the WG + Cu treatment yielded significantly more unblemished fruit (68% ± 3.61%) compared to trees treated with copper alone (55% ± 3.73% clean fruit, P < 0.01) and the untreated control trees (23% ± 2.96% clean fruit, P < 0.01) as shown in Figure 4. Trees sprayed with WG + Cu treatments every 3 weeks showed much better canker control than those only sprayed every 6 weeks (P < 0.01) as the amount of clean fruit dropped to 50% ± 3.81%. The WG + Cu spray applied every 6 weeks gave essentially the same protection as copper alone. While WG + Cu applied every 6 weeks yielded slightly less unblemished fruit, it was not significantly different from the spray treatment of Cu alone applied every 3 weeks (P = 0.106), but was significantly more effective than the untreated control (P < 0.01) which had just 23% ± 3.15% unblemished fruit.
In groves B and C, only the WG + Cu treatment sprayed every 3 weeks was applied and compared to untreated control fruit. For both areas, the WG + Cu spray was significantly better at protecting the fruit. Both Groves B and C had heavy canker pressure. In Grove B, 30% ± 4.49% of the fruit harvested after treatments with WG + Cu every 3 weeks was unblemished compared to just 15% ± 4.49% unblemished fruit in the control group (P < 0.01) (Figure 5). In grove C, the WG + Cu spray treatment yielded 48% ± 4.64% unblemished fruit, significantly more (P < 0.01) than did the untreated control which had just 30% ± 4.64% unblemished fruit (Figure 6(a)).
For 2010, melanose was assessed at only Grove C where disease pressure was moderate. Trees treated with the WG + Cu yielded 62% ± 3.84% unblemished fruit compared to the untreated control (P < 0.01) that had 53% ± 3.84% clean fruit (Figure 6(b)).
3. DISCUSSION
Both citrus canker and melanose are serious diseases of citrus that impact volume of fresh citrus fruit sales in Florida [1,5,7]. It is well documented that most plant diseases are dependent on weather conditions for pathogen survival and transport of inoculum [21]. For both citrus canker and melanose, warm temperatures and rain are necessary for pathogen release, and wind for good disease spread [2,4,6]. These weather events also stimulate flushes of new growth on citrus trees, especially on young trees. Mature grapefruit trees are also more likely to flush out than other older citrus trees [1,2]. These new flushes are difficult to keep protected in rain events as they may be unprotected (unsprayed) from disease if the flush occurred between sprays. This is an ongoing problem and makes it difficult to measure the success of a protectant when vulnerable tissues are not even covered.
Typically in Florida, copper hydroxide (Cu) is sprayed at least every 21 days (3 weeks) throughout the growing season. The success of Cu in controlling both citrus canker and melanose is generally not very high, especially in years of heavy disease pressure [6]. Evidence of this can be seen in studies presented with the increased volume of fruit that is yearly lost to canker and melanose indicating that inoculum in the field is not being controlled [4-6]. Copper hydroxide, such as the Cu used in these studies, is a wettable powder (WP) which forms an unstable suspension when mixed with water and dries to a powder

Figure 4. Percent of canker free (unblemished) grapefruit harvested in 2010 from grove A after treatments of WashGard® + Copper (WG + Cu/3Wk) or Copper only (Cu/3Wk) sprays applied every 3 weeks, Wash-Gard® + Copper applied every 6 weeks (WG + Cu/6Wk), and unsprayed control.
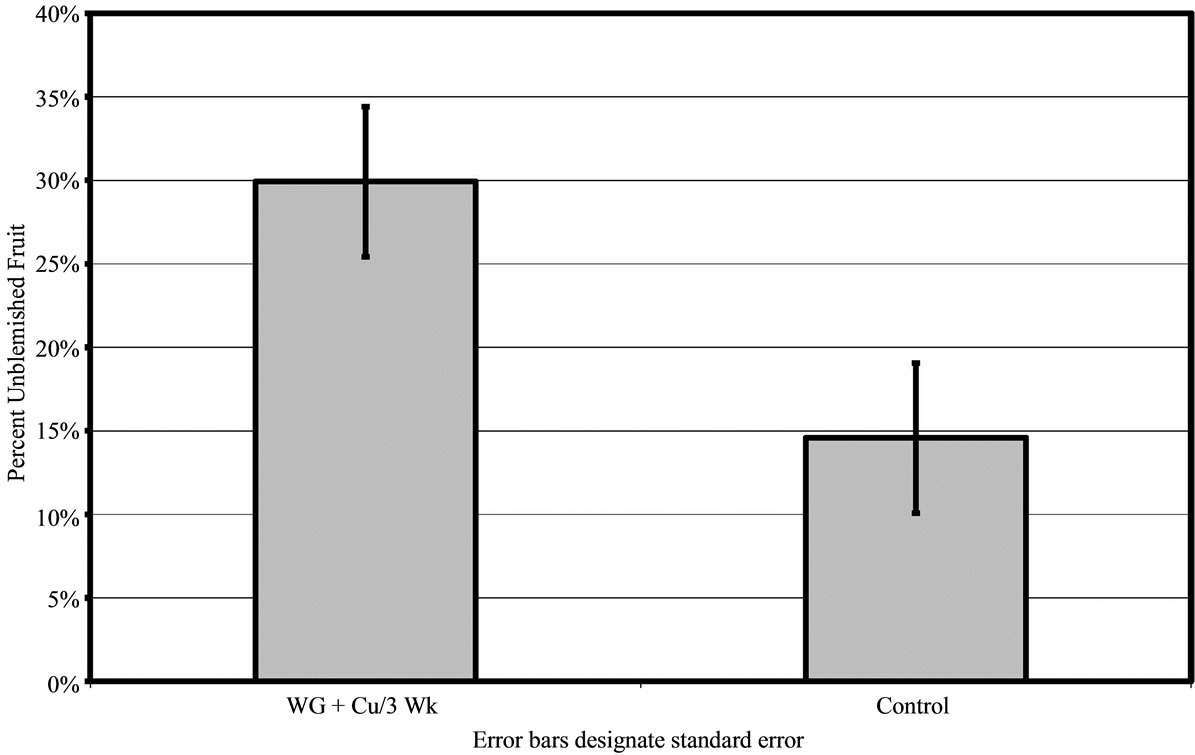
Figure 5. Percent of canker free (unblemished) grapefruit harvested in 2010 from grove B after treatment of WashGard® + Copper (WG + Cu/3 Wk) applied every 3 weeks compared to unsprayed control.
like consistency when sprayed on leaves and fruit [15,17, 18]. Studies show that many sprays, especially those composed of WP, are easily eroded by environmental and biological factors such as rain, UV light, and insect damage. Von Brugger et al. [15] showed that WP formulations were readily washed off by rain and in the case of Cu, increases in rain acidity removes it much faster. Usually for WP treatments, disease control decreased as time between application and infection increased [18]. Vincent et al [17] and Kudsk et al. [11] found that after a rain, Cu was always reduced and had poor persistence: in fact, these studies showed that Cu was one of the least effective protectants in a wet, warm climate [17]. Mondal et al. [18] found that Cu sprayed pre-infection offered protection for only about 2 days in warm, wet conditions and when sprayed post-infection, offered no protection. He postulated that for groves to have the best protection using Cu, trees would have to be sprayed twice a week, an action that is neither economically feasible nor environmentally responsible.
Data from studies presented here showed similar results, with end of season assessments in 2009 showing no differences in blemish free fruit between Cu sprays alone and control fruit (Figure 2). Data presented show that using WashGard® (WG) as a persistent carrier for Cu sprays for citrus canker significantly reduced the incidence of citrus canker, and melanose as well, compared to copper sprays alone and the control (Figure 2). The mechanism of activity of the WG not only holds the Cu onto the trees much longer than Cu alone, but it has been found that the WG absorbs moisture and keeps the Cu available to the pathogen when it is most active during periods of warm, rainy weather (data not shown). One of the most important qualities of a successful protectant is that it be present and active during periods when disease pressure is high [21,22]. Data show that WG + Cu meets the criteria of a successful protectant.
To be effective, treatments need to form a protective barrier between the plant and the pathogen [14]. When considering protection for plants, 2 factors need to be taken into account: what will stick to the leaf at the time of application and what remains as residue after weathering and expansion of tissues [12,14,15]. One important factor influencing efficiency of sprays, in addition to rain fastness, is leaf and fruit topography [12-14,20,29]. For example, deposition of droplets from sprays, applied to plant surfaces that are pubescent or waxy have less droplet retention than smooth, non-waxy surfaces [11,12, 27].
Adjuvants (e.g. oils) do not always increase persistence, but do improve the uniformity of application [11, 21,22,29]. However, other adjuvants, such as latex and wax, can increase uniformity of treatment applications as well as increase persistence [11]. In the studies with WG, the significant increase of blemish free fruit and reducetion of lesions on leaves treated with WG + Cu show that both spray application and persistence were better when compared to controls or Cu sprays alone.
Many studies show that in addition to leaf/fruit topography, droplet size and affinity of particles and drops to
 (a)
(a)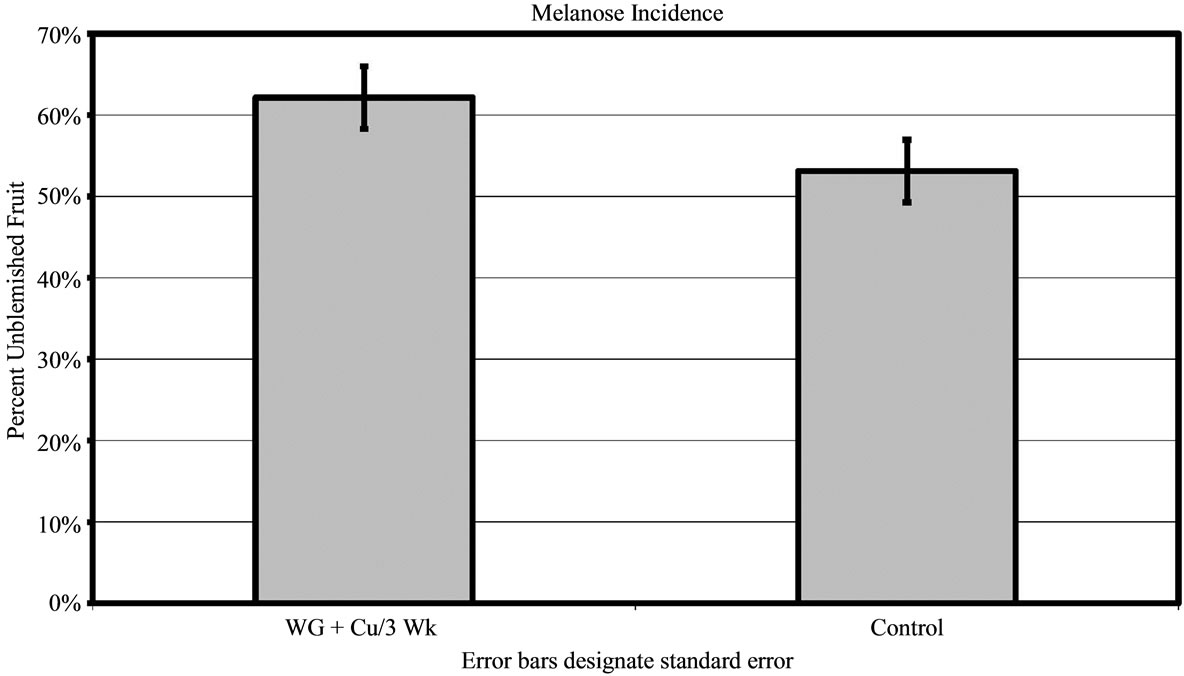 (b)
(b)
Figure 6. Percent of grapefruit free of blemishes from Canker (a) and Melanose (b) harvested from grove C in 2010 after WashGard® + Copper application every 3 weeks (WG + Cu/3 Wk) compared to Control.
each other increases treatment persistence [11-13,20]. Kudsk et al. [11] found that persistence in terms of rain fastness is inversely related to particle size: smaller particles stay in suspension better and have greater affinity to the plant surface [11]. This strong affinity resists mechanical abrasion by high intensity rains which are more damaging than longer periods of light rain [11,16].
In this study, t he adsorption and accumulation of WG + Cu could be seen on the plant surface throughout the growing season (Figure 7). The sprayer was set on smallest droplet size and light coatings were repeatedly applied. Dark, plastic covered papers were hung in the interior of the canopy to monitor efficiency of application and droplet size. Interior leaves and fruit were amply covered and the drop size was very small. Because of its relationship with WG, the Cu was maintained on the surface of the plant while copper sprays alone dried to a powdery consistency and were easily removed by dissolution and mechanical abrasion. Data from the present studies support other field trials where it was found that treatments were more persistent when applied with frequent several light sprays rather than heavy applications

Figure 7. Young grapefruit tree showing canker lesions on new, unprotected growth (flush) compared to older leaves protected with WG + Cu.
at longer intervals [10,11,22,30].
The preharvest use of film forming compounds is documented [9,10,23]. These compounds are used for sunburn protection as well as anti-transpirants [9,10,23] and form a physical barrier between bacteria, fungi and insects and the plant, protecting the plant surface. In addition to forming physical barriers, coating compounds limit free films of water necessary for cell survival and spore germination and prevent cell/spore adhesion [9, 10]. Walters [10] found that the presence of coatings can disguise clues from the plant that initiate infection and can interfere with enzyme production by the pathogen. Data show that both melanose and canker were reduced on tissues with WG + Cu and it was observed that damage by leaf miners (Phyllocnistis citrella), a common pest on citrus, was reduced on treated leaves as well. This barrier to this insect is important because leaf miner injury provides an entrance for canker bacteria into the leaf.
Initial studies with WG included sprays with WG and no copper additive. Data from these studies show that using WG alone during periods of heavy canker and melanose disease pressure did not significantly differ from the control (data not shown). However, while WG + Cu is not an efficient mechanical barrier to the canker bacteria, its synergistic activity significantly increased numbers of unblemished and marketable fruit. Our results support Walters’s hypothesis: the presence of a coating can extend the efficiency of agricultural protective sprays [10].
4. CONCLUSIONS
Citrus canker continues to be a critical problem for the Florida fresh citrus market, especially for grapefruit. Copper hydroxide protective sprays are the Industry’s sole control for canker. These prophylactics do not significantly reduce new canker formations, therefore perpetuating the spread of the disease. There are no resistant varieties and newly planted trees must be protected.
Data presented in this study show that combining the copper hydroxide with WashGard®, a carnauba based protectant, infections resulting in citrus canker and melanose can be significantly reduced. The data suggest that using WG for consecutive years will lower infections, logically reducing inoculum densities in the groves to a level where most fruit will be unblemished.
There are still studies to be undertaken when considering the use of coatings as disease protectants in the field. Field spray coatings need to be made more elastic or self-healing to allow better protection as the tissue expands. Also, it must be kept in mind that some coatings can alter the temperatures of the plant tissues so physiological parameters of the fruit should be monitored. If used prudently, a preharvest coating combined with an appropriate protectant can provide effective and persistent protection for field fruit if applied before infection.
5. DECLARATION
This article is a US Government work and is in the public domain in the USA. Mention of a trademark or proprietary product is for identification only and does not imply a guarantee or warranty of the product by the US Department of Agriculture. The US Department of Agriculture prohibits discrimination in all its programs and activities on the basis of race, color, national origin, gender, religion, age, disability, political beliefs, sexual orientation, and marital or family status.
![]()
![]()
REFERENCES
- Brunings, A.M. and Gabriel, D.W. (2003) Xanthomonas citri: Breaking the surface. Molecular Plant Pathology, 4, 141-157. doi:10.1046/j.1364-3703.2003.00163.x
- Das, A.K. (2003) Citrus canker: A review. Journal of Applied Horticulture, 5, 52-60.
- McGuire, R.G. (1988) Evaluation of bactericidal chemicals for control of Xanthomonas on citrus. Plant Disease, 72, 1016-1020. doi:10.1094/PD-72-1016
- Schubert, T.S., Rizvi , S.A., Sun X., Gottwald, T.R., Graham, J.H. and Dixon, W.N. (2001) Meeting the challenge of eradicating citrus canker in Florida, again. Plant Disease, 85, 340-356. doi:10.1094/PDIS.2001.85.4.340
- Agostini, A., Bushong, P.M., Bhatia, A. and Timmer, L.W. (2003) Influence of environmental factors on severity of citrus scab and melanose. Plant Disease, 87, 1102-1106. doi:10.1094/PDIS.2003.87.9.1102
- Timmer, L.W., Garnsey, S.M. and Graham, J.H. (2000) Compendium of citrus diseases. 2nd Edition, APS Press, St. Paul.
- Timmer, L.W. and Kucharek, T.A. (2009) Melanose. EDIS. University of Florida, IFAS Extension, Fort Pierce, 150.
- Mondal, S.N., Agostini, J.P., Zhang, L. and Timmer, L.W. (2004) Factors affecting pycnidium production of Diaporthe citri on detaches citrus twigs. Plant Disease, 88, 379-382. doi:10.1094/PDIS.2004.88.4.379
- Ziv, O. and Frederiksen, R.A. (1983) Control of foliar diseases with epidermal coating materials. Plant Disease, 67, 212-214. doi:10.1094/PD-67-212
- Walters, D.R. (2006) Disguising the leaf surface: The use of leaf coatings for plant disease control. European Journal of Plant Pathology, 114, 255-260. doi:10.1007/s10658-005-5463-7
- Kudsk, P., Mathiassen, S.K. and Kirknel, E. ( 1991) Influence of formulations and adjuvants on the rainfastness of maneb and mancozeb on pea and potato. Pesticide Science, 33, 57-71. doi:10.1002/ps.2780330107
- Rich, S. (1954) Dynamics of deposition and tenacity of fungicides. Phytopathology, 44, 203-213.
- Van Zyl, S.A., Brink, J.C., Calitz, F.J. and Fourie, P.H. (2010) Effects of adjuvants on deposition efficiency of fenhexamid sprays applied to Chardonnay grapevine foliage. Crop Protection, 29, 843-852. doi:10.1016/j.cropro.2010.04.017
- Suheri, H. and Latin, R. X. (1991) Retention of fungicides for control of Alternaria leaf blight of muskmelon under greenhouse conditions. Plant Disease, 75, 1013- 1015. doi:10.1094/PD-75-1013
- Van Bruggan, A.H.C., Osmeloski, J.F. and Jacobson, J.S. (1986) Effects of simulated acidic rain on wash-off fungicides and control of late blight on potato leaves. Phytopathology, 76, 800-804. doi:10.1094/Phyto-76-800
- Neely, D. (1970) Persistence of foliar protective fungicides. Phytopathology, 60, 1583-1586. doi:10.1094/Phyto-60-1583
- Vincent, A., Armengol, J. and Garcia-Jimenez, J. (2007) Rain fastness and persistence of fungicides for control of Alternaria brown spot of citrus. Plant Disease, 91, 393- 399. doi:10.1094/PDIS-91-4-0393
- Mondal, S.N., Vincent, A., Reis, R.F. and Timmer, L.W. (2007) Efficacy of pre and post-inoculation application of fungicides to expanding young citrus leaves for control of melanose, scab and Alternaria brown spot. Plant Disease, 91, 1600-1606. doi:10.1094/PDIS-91-12-1600
- Mondal, S.N., Vincent , A., Reis, R.F. and Timmer, L.W. (2007) Saprophytic colonization of citrus twigs by Diaporthe citrus and factors affecting pycnidial production and conidial survival. Plant Disease, 91, 387-392. doi:10.1094/PDIS-91-4-0387
- Hess, D.F. and Falk, R.H. (1990) Herbicide deposition on leaf surfaces. Weed Science, 38, 280-288.
- Walker, J.C. (1957) Plant pathology. McGraw-Hill, New York.
- Horsfall, J.G. (1956) Principles of fungicidal action. McGraw Hill, New York.
- Han, J. (1990) Use of antitranspirant epidermal coatings for plant protection in China. Plant Disease, 74, 263-266. doi:10.1094/PD-74-0263
- Hintze, J.L. (2006) Number crunching statistical systems. Kayesville.
- Rao, P.V. (1998) Statistical methods in the life sciences. Duxbury Press, Pacific Grove.
- Duveiller, E. (1994) A pictorial series of disease assessment keys for bacterial leaf streak of cereals. Plant Disease, 78, 137-141. doi:10.1094/PD-78-0137
- Pfender, W.F. and Eynard, J. (2009) Field assessment of a model for fungicide effects on intraplant spread of stem rust in perennial ryegrass seed crops. Phytopathology, 99, 696-703. doi:10.1094/PHYTO-99-6-0696
- Jorgensen, L.N., Seeker , B.J.M. and Nielsen, G.C. (1996) Monitoring diseases of winter wheat in both a field and a National level in Denmark. Crop Protection, 15, 383-390. doi:10.1016/0261-2194(96)00009-9
- Martin, J.T. and Juniper, B.E. (1970) The cuticles of plants. St. Martin’s Press, New York.
- Juste, F., Sanchez, S., Ibanez, R., Val, L. and Garcia, C. (1990) Measurement of spray deposition and efficiency of pesticide application in citrus orchards. Journal of Agricultural Engineering Research, 46, 187-196. doi:10.1016/S0021-8634(05)80125-8

