American Journal of Analytical Chemistry
Vol.06 No.04(2015), Article ID:55131,13 pages
10.4236/ajac.2015.64034
On an Expression of Extraction Constants without the Interfacial Equilibrium-Potential Differences for the Extraction of Univalent and Divalent Metal Picrates by Crown Ethers into 1,2-Dichloroethane and Nitrobenzene
Yoshihiro Kudo*, Shoichi Katsuta
Graduate School of Science, Chiba University, Chiba, Japan
Email: *iakudo@faculty.chiba-u.jp
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 17 February 2015; accepted 25 March 2015; published 27 March 2015
ABSTRACT
An idea on interfacial equilibrium-potential differences (Dfeq) which are generated for the extraction of univalent metal picrate (MPic) and divalent ones (MPic2) by crown ethers (L) into high-po- lar diluents was improved. These potentials were clarified with some experimental extraction- data reported before on the M = Ag(I), Ca(II), Sr(II) and Ba(II) extraction with 18-crown-6 ether (18C6) and benzo-18C6 into 1,2-dichloroethane (DCE) and nitrobenzene (NB). Consequently, it was demonstrated that the Dfeq values from the extraction-experimentally obtained logKD,Pic ones are in agreement with or close to those calculated from charge balance equations in many cases, where the symbol, KD,Pic, denotes an individual distribution constant of Pic− into the DCE or NB phase. Also, it was experimentally shown that extraction constants based on the overall extraction equilibria do not virtually contain the Dfeq terms in their functional expressions.
Keywords:
Extraction Constants, Distribution Constant of a Single Ion, Interfacial Equilibrium-Potential Differences, 1,2-Dichloroethane, Nitrobenzene, Metal Picrates, Crown Ethers

1. Introduction
Univalent and divalent metal picrates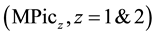 , such as alkali and alkaline-earth metal ones, have
, such as alkali and alkaline-earth metal ones, have
been extracted by crown compounds (L) into the high-polar diluents, such as 1,2-dichloethane (DCE), dichloromethane and nitrobenzene (NB) [1] -[5] . In such high-polar diluents, an extracted ion-pair complex, MLPicz, dissociates MLz+ and zPic− [1] -[3] [6] . In introducing these component equilibria in an extraction model, an individual distribution constant (KD,A) of Pic− (=A−) into the diluents has been determined extraction-experimen- tally [1] -[3] [7] . However, in spite of the limitation of the same KD,A definition and the same diluents, the thus-determined KD,Pic values have differed from each other. For example, the logKD,Pic values were −0.94 [2] for the PbPic2 extraction with 18-crown-6 ether (18C6), −1.34 [7] for the SrPic2 one with benzo-18C6 (B18C6) into NB, −2.46 [3] for the AgPic one with benzo-15-crown-5 ether, −1.89 [2] for the PbPic2 one with 18C6 and −4.35 [6] for the CdPic2 one with 18C6 into DCE. Thus, their values have changed over experimental errors with combinations of MPicz and L.
To clarify a reason for such differences, the authors have applied the idea [8] of an interfacial potential dif-
ference (Dfeq) at extraction equilibrium to an expression of log KD,A, namely 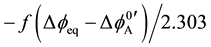 [3] [6]
[3] [6]
[7] , where the negative sign being in the front of f, which denotes F/RT, comes from the electrical charge of A−. In addition to this, extraction constants, Kex± and Kex2±, have been electrochemically expressed as
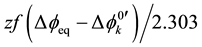 at
at 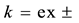 and
and 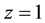 and at ex±, ex2± and 2 [3] [7] . Here,
and at ex±, ex2± and 2 [3] [7] . Here, 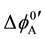 and
and 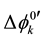 refer
refer
to standard formal potentials for the single distribution of A− into the diluent or organic (o or org) phase and the formal potentials for the overall equilibrium, respectively. Also, Kex± and Kex2± have been defined experimentally
by extraction as 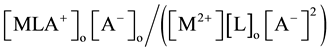 [2] [7] or
[2] [7] or 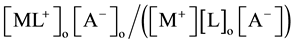 [1] -[3] and
[1] -[3] and 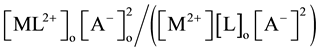 [7] , respectively.
[7] , respectively.
On the other hand, from the thermodynamic points of view, these extraction constants are resolved into 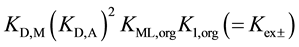 for
for  [7] ,
[7] , 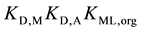 for 1 [3] and
for 1 [3] and
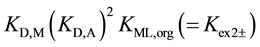 for
for  [7] . Here, the component equilibrium constants, KML,org (complex
[7] . Here, the component equilibrium constants, KML,org (complex
formation in the o phase) and K1,org (1st-step ion-pair formation in the o one), do not contain the Dfeq terms in their expressions, because the constants are of homogeneous systems that all species relevant to the reaction are present in the single o phase [3] [7] ; namely no interface is involved in these processes. Similarly, the distribution constant of Mz+ has been expressed with KD,M (see Equation (3) at  in the Section 2.1) [3] . Therefore,
in the Section 2.1) [3] . Therefore,
since KD,M and KD,A are present in the 

tually the Dfeq ones. Thereby, the extraction constants virtually lose the Dfeq terms on their functional expres-
sions. Thus, the above expression, such as

experimentally-evaluated values and theoretically-reproduced ones [7] .
In the present paper, in order to solve the above two contradictions, namely the differences of KD,A caused by experimental conditions of extraction and the contradiction based on the thermodynamic cycles [3] [7] , we proposed another expression without Dfeq of the extraction constants, Kex± and Kex2±. In course of clarifying this expression, some experimentally-determined constants [3] [7] , such as Kex±, an individual distribution constant (KD,ML) of the complex ion ML2+ into the NB phase and that of AgL+ into DCE, were also reproduced by calculation. Here, the AgPic and MPic2 (M = Ca, Sr & Ba) extraction with L = 18C6 and/or B18C6 [3] [7] were employed as model systems. Also, a meaning of the Dfeq values [3] [7] & [8] which were calculated from the logKD,A ones determined by the extraction experiments was discussed based on an electroneutrality-point of view [8] for the o phases. Moreover, the thus-obtained expressions for the extraction constants were applied to other types of extraction systems with o = DCE and NB.
2. Theory
2.1. Dfeq Values Derived from Charge Balance Equations for the o Phase
(i) Case of the M(I) extraction with L. For the extraction equilibrium, 

for the o phase. The concentrations of M+ and A− in the o phase were modified as

by using electrochemical equations [6] [8] such as

and

see Appendix B in ref [6] for a detailed derivation from electrochemical potentials to this equation. Here, 




with


and

Accordingly, the following equation is derived.

Hence, if the [M+], [ML+]o and [A−] values are determined experimentally, then we can obtain the Dfeq values from Equation (6) immediately; the [ML+]o values were calculated here from the relation



(ii) Case of the M(II) extraction with L. Similarly, we can consider the following stepwise extraction-equili- bria [6] [12] at the same time: 

As described above, this equation was modified to [8]

Defining as 

with


and

We can exactly solve this equation for x based on the mathematical formula [13] . Its real solution is

where 

The b¢ values were evaluated from the relation, 





extraction experiments [2] [7] and also we were able to calculate the other values in r+ from the experimental data [7] .
2.2. On Expressions of the Extraction Constants without Dfeq
According to previous papers, the two of the three extraction constants have been defined as 





These two kinds of extraction constants contain the Dfeq terms as parameters in their functional expressions[3]
[7] . On the other hand, logKex has been expressed as 


In the above functions, some contradictions have been observed in the former cases: see Appendix in ref. [7] . As an example similar to that described in the introduction, the relation,

the resulting component equilibrium-constant K2,org does not relate with Dfeq [7] ; namely K2,org and Kex are the constants at

comes 




In order to cancel such contradictions, we assume here that the two extraction constants are functions without Dfeq, as well as that of Kex [3] [7] . Accordingly, the constants are defined as

and

That is, by our traditional sense, it is proposed here that complicated equilibrium constants, such as Kex, Kex± and Kex2±, do not contain the Dfeq terms in their functions. This means that these constants are ordinarily defined without Dfeqor under the condition of 


The relations in Table 1 shows that the individual distribution process of A− [12] cancels out that of a cation [14] , such as M+, R4N+, M2+ and ML2+, in Dfeq. As an example, the thermodynamic relation for M(II)

can be rearranged into

Table 1. Relations between Kex± or Kex2± and its component equilibrium constants and their corresponding 
ak = ex±, ex2±, ML,org, ML,w, & 1,org, where the symbol “w” shows a water phase; bThermodynamic cycle; cRef. [3] ; dRef. [7] .
Therefore, the relation (c) in Table 1 is immediately obtained. From Equations (2) and (8), one should obviously see that Dfeq of KD,M equals that of KD,A in the extraction system of Equation (13). Also, we can rewrite Equation (13) to

Consequently, Equation (14) or (13) does not contain the Dfeq term and is virtually expressed with only the standard formal potentials (at
3. Results and Discussion
3.1. On a Meaning of Dfeq Estimated from log KD,A
Table 2(a) lists fundamental data [3] for the extraction of AgPic by B18C6 into DCE. The Dfeq values were calculated from Equation (4) and the experimental log KD,Pic values in Table 2(a).
Here, 
Table 2. (a) Fundamental data for the extraction of AgPic by B18C6 into DCE at 298 K; (b) Evaluated Dfeq values; (c) Reproduced logKex± values; (d) Evaluated 
aValues calculated from 

*
*

Also, we estimated Dfeq,av from Equation (6) with Equation (5), where Dfeq,av denotes an average value for each run.
The both values, expressed as 

Average I values of the extraction systems in Table 2(a) were 0.0036 mol×dm−3 for the no. 1A [3] , 0.0028 for 1B, 0.0027 for 1C and 0.097 for 2; I denotes the ionic strength of the water phase in the extraction. Except for the data no. 2, we can handle other three data on the average, because experimental conditions [3] of the data are essentially the same (see the footnote g in Table 2(a) for no. 2). So the following values were obtained at 298 K and L = B18C6: logKex± = 0.31 ± 0.14 and logKD,Pic = −2.54 ± 0.07;

in the same IDCE range. The symbol, IDCE, refers to the average ionic strength of the DCE phase; the same is true of INB (see Table 3).
Table 3(a) summarizes the fundamental data [7] for the extraction of MPic2 (M = Ca, Sr & Ba) by 18C6 and B18C6 into NB.
The Dfeq values were calculated from Equation (4) with the logKD,Pic values in Table 3(a) and the

Dfeq,av values were estimated in the same manner. The above findings are listed in Table 3(b).
For the 18C6 extraction systems, the 

Except for the 

cial equilibrium-potential differences, Dfeq, based on Equation (4) are essentially the same as those based on
Equation (6). The differences between 

charge balance equation between extraction experiments (see Appendix II) and electrochemical (or theoretical)
treatments, namely 


were the maximum of the B18C6- and 18C6-M(II) extraction systems. Practically, the 

On the basis of the above facts, 








3.2. Experimental Proof of Kex± and Kex2± without Dfeq
We obtained the log Kex± values of the AgPic extraction with B18C6 into DCE from the relation (a) in Table 1 with 


ponding logKML,DCE value in Table 2(a). These values, expressed as 
The KD,AgL calculation can be an indirect proof of Kex± without Dfeq. First, the log KD,AgL values (namely 






Next, the logKD,AgL values were reproduced by using the equation,




Table 2(d) are in good accordance with the values listed in Table 2(a). Thus the log KD,AgL values can be well
reproduced. From the results of 

Pic-B18C6 extraction system.
Moreover, an average 




1A and 1B were close to those in Table 2(a).
The logKex± values for the M(II)-B18C6 extraction into NB were calculated from the relation (c) in Table 1.
These 
been determined by the procedure [2] [7] described in Appendix II. This accordance indicates that Equation (11)
without Dfeq is satisfied. In this calculation, 

[10] , logKCaL,NB = 11.2, logKSrL,NB = 13.1, logKBaL,NB = 13.4 for L = 18C6 [17] , logKCaL,NB = 9.43, logKSrL,NB = 11.1 and logKBaL,NB = 11.6 for L = B18C6 [17] were employed. Also, the logKD,M values were calculated from
the modified form of Equation (3), 


The following discussion is similar to that from 



ues were 0.48 for the Ca-18C6 [18] and -B18C6 [19] systems, 2.72 [20] for Sr-18C6, 3.87 [20] for Ba-18C6, 2.41 [15] for Sr-B18C6 and 2.90 [13] for Ba-B18C6. Also, 

The calculated 
As similar to 
without Dfeq. Then, the logKD,ML values at 298 K were estimated from the 


The thus-calculated 
logKD,ML values in Table 3(a) have been calculated from the relation (d) in Table 1 [7] . This fact indicates that Equation (12) satisfies indirectly the thermodynamic cycle of (f).
Table 3. (a) Fundamental data for the extraction of MPic2 (M = Ca, Sr& Ba) by L into NB at 298 K.a; (b) Evaluated Dfeq values; (c) Reproduced logKex± values; (d) Evaluated 
aRef. [7] ; bUnit: mol dm−3; c
*
*

The above calculation results for the AgPic and MPic2 extraction with L indicate that the assumption of Equations (11) and (12) without Dfeq is essentially valid. In other words, the overall extraction constants, Kex± and Kex2±, must be expressed rationally as functions without Dfeq.
3.3. For Applications to Other Extraction Systems
The above handling based on Table 1 can be also applied to the practical extraction equilibria of





and

As examples, thermodynamic points of view suggest the following cycles for the above equilibria:




with 


and

with


functions with the Dfeq ones.
The relation, 












Similarly, KD,T cancels out KD,A in (E13c), where T- denotes another anion. That is,

equilibria, (E14) & (E15), one can handle them in the same manner as that described above for the AgPic and MPic2 extraction with L, respectively.
We can easily see that the KD,H and KD,Pu values cancel out the KD,Cl one in (E16c). That is,






4. Conclusion
It was demonstrated that the Dfeq values calculated from the experimental logKD,Pic ones are in agreement with or close to those more-accurately done from the charge balance equations for the species with M(I) in the DCE phase and with M(II) in the NB one, except for some cases. This demonstration indicates that the plots of 


values and then the first-approximated Dfeq ones. These results will give an answer to how one explain the differences in KD,A among extraction experiments of various MA or MA2 by various L. Also, we clarified that the assumption of Equations (11) and (12) is valid for the AgPic and MPic2 extraction with 18C6 and/or B18C6. This eliminated the contradictions [3] [7] due to Dfeq from the thermodynamic cycles. Moreover, the present work indicates a possibility that the proposed handling can be applied to various extraction systems with neutral ligands at least.
Acknowledgements
The authors thank Mr. Tomohiro Amano, Mr. Satoshi Ikeda and Mr. Yuki Ohsawa for their experimental assistances.
Cite this paper
Yoshihiro Kudo, Shoichi Katsuta,, (2015) On an Expression of Extraction Constants without the Interfacial Equilibrium-Potential Differences for the Extraction of Univalent and Divalent Metal Picrates by Crown Ethers into 1,2-Dichloroethane and Nitrobenzene. American Journal of Analytical Chemistry, 06, 350-363. doi: 10.4236/ajac.2015.64034
References
- Kudo, Y., Harashima, K., Katsuta, S. and Takeda, Y. (2011) Solvent Extraction of Sodium Permanganate by Monobenzo-3m-Crown-m Ethers (m = 5, 6) into 1,2-Dichloroethane and Nitrobenzene: A Method which Analyzes the Extraction System with the Polar Diluents. International Journal of Chemistry, 3, 99-107.
http://dx.doi.org/10.5539/ijc.v3n1p99 - Kudo, Y., Takahashi, Y., Numako, C. and Katsuta, S. (2014) Extraction of Lead Picrate by 18-crown-6 Ether into Various Diluents: Examples of Sub-Analysis of Overall Extraction Equilibrium Based on Component Equilibria. Journal of Molecular Liquids, 194, 121-129.
http://dx.doi.org/10.1016/j.molliq.2014.01.017 - Kudo, Y., Ogihara, M., Katsuta, S. and Takeda, Y. (2014) An Electrochemical Understanding of Extraction of Silver Picrate by Benzo-3m-Crown-m Ethers (m = 5, 6) into 1,2-Dichloroethane and Dichloromethane. American Journal of Analytical Chemistry, 5, 433-444.
http://dx.doi.org/10.4236/ajac.2014.57052 - Valente, M., Sousa, S.F., Magalhaes, A.L. and Freire, C. (2010) Crown-Ether Type Podands as Alkali Metal Cation Extractants: Interface of the Number of Oxygens in the Chain. Journal of Solution Chemistry, 39, 1230-1242.
http://dx.doi.org/10.1007/s10953-010-9579-9 - Valente, M., Sousa, S.F., Magalhaes, A.L. and Freire, C. (2011) Complexation of Alkali Metal Cations by Crown-Ether Type Podands with Applications in Solvent Extraction: Insights from Quantum Chemical Calculations. Journal of Molecular Model, 17, 3275-3288.
http://dx.doi.org/10.1007/s00894-011-1004-9 - Kudo, Y., Horiuchi, N., Katsuta, S. and Takeda, Y. (2013) Extraction of Cadmium Bromide and Picrate by 18-crown-6 Ether into Various Less-Polar Diluents: Analysis of Overall Extraction Equilibria Based on Their Component Equilibria with Formation of Their Ion Pairs in Water. Journal of Molecular Liquids, 177, 257-266.
http://dx.doi.org/10.1016/j.molliq.2012.10.015 - Kudo, Y. and Takeuchi, T. (2014) On the Interfacial Potential Differences for the Extraction of Alkaline-Earth Metal Picrates by 18-Crown-6 Ether Derivatives into Nitrobenzene. Journal of Thermodynamics & Catalysis, 5, 6 pp.
http://dx.doi.org/10.4172/2157-7544.1000133 - Takeda, Y., Ezaki, T., Kudo, Y. and Matsuda, H. (1995) Distribution Study on Electroneutral ans Protonated Amino Acids between Water and Nitrobenzene. Determination of the Standard Ion-Transfer Potentials of Protonated Amino Acids. Bulletin of the Chemical Society of Japan, 68, 787-790.
http://dx.doi.org/10.1246//bcsj.68.787 - Marcus, Y. (1983) Thermodynamic Functions of Transfer of Single Ions from Water to Nonaqueous and Mixed Solvents: Part 1-Gibbs Free Energies of Transfer to Nonaqueous Solvents. Pure & Applied Chemistry, 55, 977-1021.
- Pivoňková, M. and Kyrs, M. (1969) The Distribution of Alkaline Earth Dipicrylaminates and Tetraphenylborates Between Water and Nitrobenzene Phases. Journal of Inorganic and Nuclear Chemistry, 31, 175-185.
http://dx.doi.org/10.1016/0022-1902(69)80066-7 - Kudo, Y., Harashima, K., Hiyoshi, K., Takagi, J., Katsuta, S. and Takeda, Y. (2011) Extraction of Some Univalent Salts into 1,2-Dichloroethane and Nitrobenzene: Analysis of Overall Extraction Equilibrium Based on Elucidating Ion-Pair Formation and Evaluation of Standard Potentials for Ion Transfers at the Interfaces between Their Diluents and Water. Analytical Sciences, 27, 913-919.
http://dx.doi.org/10.2116/analsci.27.913 - Kudo, Y., Katsuta, S. and Takeda, Y. (2012) Evaluation of Overall Extraction Constants for the Crown Ether-Complex Ions of Alkali and Alkaline-Earth Metal with Counter Picrate Ions from Water into Nitrobenzene Based on Their Component Equilibrium Constants. Journal of Molecular Liquids, 173, 66-70.
http://dx.doi.org/10.1016/j.molliq.2012.06.003 - Umegaki, T. (1983) Su-gakuYo-koKo-shiki-syu (Handbook of Mathematical Formula, 4th Edition). Komatsu, Y., Ed., Hirokawa Publishing Co., Tokyo, Chap. 3, 78-79. (In Japanese)
- Rais, J. (1976) Individual Extraction Constants of Univalent Ions in the System Water-Nitrobenzene. Collection of Czechoslovak Chemical Communications, 36, 3253-3262.
http://dx.doi.org/10.1135/cccc19713253 - Takeda, Y., Kohno, R., Kudo, Y. and Fukada, N. (1989) Stabilities in Water and Transfer Activity Coefficients from Water to Nonaqueous Solvents of Benzo-18-crown-6-Metal Ion Complexes. Bulletin of the Chemical Society of Japan, 62, 999-1003.
http://dx.doi.org/10.1246/bcsj.62.999 - Takeda, Y. (2002) Extraction of Alkali Metal Picrates with 18-crown-6, benzo-18-crown-6, and Dibenzo-18-crown-6 into Various Organic Solvents. Elucidation of Fundamental Equilibria Governing Extraction-Ability and -Selectivity. Bunseki Kagaku (Analytical Chemistry), 51, 515-525. (In Japanese)
- Kudo, Y., Miyakawa, T., Takeda, Y., Matsuda, H. and Yajima, S. (1996) Ion-Transfer Polarographic Study of the Distribution of Alkali and Alkaline-Earth Metal Complexes with 3m-crown-m Ether Derivatives (m = 6, 8) between Water and Nitrobenzne Phases. Journal of Inclusion Phenomena and Molecular Recognition in Chemistry, 26, 331-341.
http://dx.doi.org/10.1007/BF01053550 - Hoiland, H., Ringseth, J.A. and Brun, T.S. (1979) Cation-Crown Ether Complex Formation in Water. II. Alkali and Alkaline Earth Cations and 12-crown-4, 15-crown-5, and 18-crown-6. Journal of Solution Chemistry, 8, 779-792.
http://dx.doi.org/10.1007/BF00648577 - Takeda, Y. and Nishida, M. (1989) Solvent Extraction of Various Metal Picrates with Benzo-18-crown-6 into CHCl3. Bulletin of the Chemical Society of Japan, 62, 1468-1471.
http://dx.doi.org/10.1246/bcsj.62.1468 - Izatt, R.M., Terry, R.E., Haymore, B.L., Hansen, L.D., Dalley, N.K., Avondet, A.G. and Christensen, J.J. (1976) Calorimetric Titration Study of the Interaction of Several Uni- and Bivalent Cations with 15-crown-5, 18-crown-6, and Two Isomers of Dicyclohexo-18-crown-6 in Aqueous Solution at 25°C and μ= 0.1. Journal of the American Chemical Society, 98, 7620-7626.
http://dx.doi.org/10.1021/ja00440a028 - Iwachido, T., Minami, M., Sadakane, A. and Toei, K. (1977) The Coextraction of Water into Nitrobenzene with Alkaline Earth Metal 2,2',4,4',6,6'-Hexanitrodiphenylaminats in the Presence of the Crown Ethers. Chemistry Letters, 6, 1511-1514.
http://dx.doi.org/10.1246/cl.1977.1511 - Kusakabe, S. and Arai, M. (1996) Solvent Extraction of Several Anions with Tetrabutylammonium Ion into 1,2-Dichloroethane and Hydration of Anions in the Organic Phase. Bulletin of the Chemical Society of Japan, 69, 581-588.
http://dx.doi.org/10.1246/bcsj.69.581 - Katsuta, S., Wakabayashi, H., Tamaru, M., Kudo, Y. and Takeda, Y. (2007) Tetraalkylammonium Picrates in the Dichloromethane-Water System: Ion-Pair Formation and Liquid-Liquid Distribution of Free Ions and Ion Pairs. Journal of Solution Chemistry, 36, 531-547.
http://dx.doi.org/10.1007/s10953-007-9121-x - Katsuta, S. (2013) Distribution Behavior of Neutral and Anionic Compounds in Ionic Liquid/Water Biphase Systems. Bunseki Kagaku (Analytical Chemistry), 62, 297-315. (In Japanese)
- Katsuta, S., Nakamura, K., Kudo, Y. and Takeda, Y. (2012) Mechanisms of Rules of Anion Partition into Ionic Liquids: Phenolate Ions in Ionic Liquid/Water Biphasic Systems. Journal of Physical Chemistry B, 116, 852-859.
http://dx.doi.org/10.1021/jp210444n - Camagong, C.T. and Honjo, T. (2001) Separation of Gold (III) as Its Ion-Pair Complex with 18-Crown-6 from Hydrochloric Acid Media by Means of Solvent Extraction. Analytical Sciences, 17, i725-i728. [Proceedings of IUPAC International Congress on Analytical Sciences 2001 (ICAS 2001)]
- Dernini, S., Scrugli, A., Palmas, S. and Polcaro, A.M. (1996) Competitive Extraction of Pb2+ by Dicyclohexano-18-crown-6 from Heavy Metal Aqueous Solutions. Journal of Chemical & Engineering Data, 41, 1388-1391.
http://dx.doi.org/10.1021/je9601523 - Mohapatra, P.K. and Manchanda, V.K. (1996) Ion-Pair Extraction of Tetravalent Plutonium from Hydrochloric Acid Medium Using Crown Ethers. Journal of Inclusion Phenomena and Molecular Recognition in Chemistry, 25, 257-265.
http://dx.doi.org/10.1007/BF01044996 - Yoshida, Y., Yoshida, Z., Aoyagai, H., Kitatsuji, Y., Uehara, A. and Kihara, S. (2002) Evaluation of Gibbs Free Energy for the Transfer of a Highly Hydrophilic Ion from an Acidic Aqueous Solution to an Organic Solution Based on Ion Pair Extraction. Analytica Chimica Acta, 452, 149-161.
http://dx.doi.org/10.1016/S0003-2670(01)01436-2 - Markin, V.S. and Volkov, A.G. (1989) The Gibbs Free Energy of Ion Transfer between Two Immiscible Liquids. Electrochimica Acta, 34, 93-107.
http://dx.doi.org/10.1016/0013-4686(89)87072-0 - Kudo, Y., Usami, J., Katsuta, S. and Takeda, Y. (2004) Solvent Extraction of Silver Picrate by 3m-crown-m Ethers (m = 5, 6) and Its Mono-Benzo-Derivative from Water into Benzene or Chloroform: Elucidation of an Extraction Equilibrium Using Component Equilibrium Constants. Talanta, 62, 701-706.
http://dx.doi.org/10.1016/j.talanta.2003.09.022
Appendix I
The basic extraction model [1] [3] [31] for the case (i) is as follows.





and

Consequently, these component equilibria yield those of 




[Pic-] calculation. The distribution [31] of 
The case (ii) [2] [6] [7] was







and

where the distribution of 



The both models, (i) & (ii), do not contain supporting electrolytes in the o phases. This point is a large difference from corresponding electrochemical measurements [29] [30] .
Appendix II
The KD,A values have been determined extraction-experimentally using the following equations [1] -[3] [6] [7] .


for 



versus 

give the KD,A value with the Kex ones for the MA- and MA2-L extraction systems, respectively. Here, the

calculated by a successive approximation [1] -[3] [6] [7] . The following mass-balance equations have been
employed for the approximation: 

[6] [7] against Equation (7) (see the Section 3.1).
Similarly, the Kex± values have been evaluated from the other arranged form of Equation (A15),

for 

NOTES
*Corresponding author.







