Natural Science
Vol.5 No.12(2013), Article ID:41052,11 pages DOI:10.4236/ns.2013.512149
Diet breadth variation and trophic plasticity behavior of the African bonytongue Heterotis niloticus (Cuvier, 1829) in the Sô River-Lake Hlan aquatic system (Benin, West Africa): Implications for species conservation and aquaculture development
![]()
1PRECOB, Unité de Recherches sur les Zones Humides, Département de Zoologie, Faculté des Sciences et Techniques, Université d’Abomey-Calavi, Cotonou, Bénin; *Corresponding Author: alphonseadite@gmail.com
2Département de Physiologie Animale, Laboratoire de Pharmacologie, Faculté des Sciences et Techniques, Université d’AbomeyCalavi, Cotonou, Bénin
3Faculté d’Agronomie, Université de Parakou (UNIPAR), Parakou, Bénin
Copyright © 2013 Alphonse Adite et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2013 are reserved for SCIRP and the owner of the intellectual property Alphonse Adite et al. All Copyright © 2013 are guarded by law and by SCIRP as a guardian.
Received 15 October 2013; revised 15 November 2013; accepted 23 November 2013
Keywords: African Bonytongues; Aquaculture; Conservation; Diet Breadth; Foraging Behavior; Gill Raker; Omnivore; Trophic Plasticity
ABSTRACT
The African bonytongue, Heterotis niloticus (Pisces: Osteoglossidae), is an omnivore foraging mainly on aquatic insects, microcrustacea, seeds and detritus. We examined the diet breadth and the trophic plasticity behavior of this species (1461 specimens) in the Sô River and Lake Hlan water system located in the southern Benin (West Africa). Overall, the mean diet breadths of the two populations of Heterotis from both habitats were not significantly (p ≥ 0.05) different and were not associated with seasons. However, in Lake Hlan, mean diet breadths tended to increase with size (r = 0.81) and gut length (r = 0.82) indicating that bonytongues ingest a broader range of food resources as they grow. In both habitats, the positive correlation of both standard length (Log SL) and gut length (Log GL) with the volumetric proportions of detritus and with the volumetric proportions of seeds suggests that the consumption of these two food resources increased with the size of Heterotis and with the development of the digestive tract. Likewise, the negative correlation of both (Log SL) and (Log GL) with the volumetric proportions of aquatic insects and with the volumetric proportions of microcrustacea suggests that the consumption of these two food categories decreased as the size and the gut length of Heterotis increased. The differences in the consumption of microcrustacea (13.77% in Lake Hlan versus 2.63% in Sô River) and mollusks (0.73% in Lake Hlan versus 4.91% in Sô River) evidenced that Heterotis shifts his trophic structure according to resource availability in the habitat. This foraging behavior suggests a degree of trophic plasticity in Heterotis. The specialized morphological structure of Heterotis, mainly the presence of a relatively high number of gill rakers (42 - 94 rakers on the first branchial arch) during its whole life, allowing sieving of zooplankton and other microcrustacea, and the presence of the gizzard favored this trophic plasticity. The broader diet breadth coupled with the trophic plasticity behavior is probably an advantage because it enables Heterotis not only to colonize and to adapt to unstable and changing aquatic habitats, but also to invade and to well-establish in various ecosystems, such as freshwater lakes, swamps, inundated plains, streams, rivers and fish farming ponds. As a result, the wider diet breadths and the trophic plasticity behavior depicted are useful eco-ethological tool for the conservation and the aquaculture development of H. niloticus.
1. INTRODUCTION
The Osteoglossiformes are highly specialized teleostean fishes of the Osteoglossomorpha super order with species almost wholly confined to tropical freshwater systems [1-3]. Among them, Heterotis niloticus (Osteoglossidae) is the only species of bonytongue occurring in Africa freshwater systems [4-6]. Indeed, Heterotis is widely distributed in the Nilo-Soudanian region and the Congo region of Central Africa and occurs in rivers such as Senegal, Gambia, Niger, Nile, Volta, Oueme, Lake Tchad, and others natural lakes and wetlands [7-9]. Moreover, Heterotis has been introduced in many lakes and aquaculture centers (e.g. lake Kossou and Ayame in Ivory coast, Lake Nyong in Cameroon) [6,10].
In Benin, Heterotis niloticus is widely distributed in rivers and inundated plains (Niger, Oueme, Zou, Sô, Mono, Couffo etc.), and freshwater lakes (Toho-Todougba, Nokoue, Hlan, Toho, lagoon of Porto-Novo, Azilli, Nakava, Cele, Codo etc.) [11-13]. The species is intensively exploited by both commercial and subsistence fishermen, and therefore, constitutes an important species for artisanal fisheries. Annual capture of Heterotis reached 742 tons and valued about $1,485,000 [13].
Heterotis has been characterized as a microphage or detritivore [14-16]. Recent studies [13] reveal that the African bonytongue is an omnivore foraging mainly on detritus, seeds, microcrustacea and aquatic insects.
The ability of a fish species to exploit a broad range of food resources (high diet breadth) or low range of food resources (weak diet breadth) may result from a specialized morphological structure (e.g. dental morphology, body form, number and structure of gill raker, intestine length etc.). For example the African pike, Hepsetus, a top carnivorous species [12] with high-developed teeth exhibits a very low diet breadth. On the contrary, Clarias gariepinus, a predator-omnivore species with less developed dental structure but with a relatively high number of small gill raker forages on a relatively high number of food resources, thus exhibiting a high diet breadth [14].
In addition to the morphological structure, the spatial and seasonal variation in the availability of food resources may greatly affect the food ingested and diet breadth, leading species to exhibit a trophic plasticity. Because Heterotis colonizes many natural aquatic systems ranging from rivers, streams to natural lakes and swamps [17], knowledge on diet breadth and trophic plasticity would give a better insight into the trophic structure dynamic, an important tool and baseline information to conserve, manage and restore fish resources in their habitat and to develop aquaculture technologies [18,19]. Given the spatial and seasonal variation of the food resources availability, it is important to well document the diet breadth variation and the trophic plasticity to explore the species feeding strategies.
The present study aims to investigate the variation of the diet breadth in relation to space and season and the trophic plasticity behavior in order to better document the trophic structure dynamic in the Sô River and Lake Hlan system.
2. MATERIAL AND METHODS
2.1. Study Locations
Field surveys were conducted at two locations in southern Benin (Figure 1): a reach of the lower Sô River (6˚34.97 N; 2˚23.75 E) and Lake Hlan (6˚56.88 N; 2˚19.48 E). This region has a sub-equatorial climate with two wet seasons (April to July; mid-September to October) and two dry seasons (November to mid-March; August to mid-September). Annual rainfall recorded for 2002 was 1167.2 mm [20], and the highest monthly rainfall during the investigation was 438.8 mm for June 2002. The Sô River is a secondary channel of the lower Oueme River and flows southward, parallel with the Oueme, approximately 100 km through a floodplain covering approximately 1000 km2 [12]. Hydrology of rivers in southern Benin is strongly influenced by seasonal rainfall in the northern region. During the peak flood season (October-November), water from the Oueme and Sô rivers covers extensive floodplains. Habitat parameters were characterized at each sampling site (water quality, aquatic vegetation, substrate, riparian vegetation, and land use).

Figure 1. Map showing Benin in Africa (a), the study region in southern Benin (b) and the sampling sites (dots) in Sô River floodplain (villages Ahome-Gblon, Ahome-Lokpo Zoungome, Kinto) and Lake Hlan from South to North, respectively.
Water depth was measured using a calibrated rope. Temperature and dissolved oxygen were measured to the 0.1˚C and 0.1 mg·l−1 respectively with a digital oxythermometer. Turbidity was measured to the nearest millimeter with a secchi disk. pH was measured to the nearest 0.1 with a portable pH meter. Salinity, total dissolved solids (TDS) and conductivity were measured to the nearest 1, 0.1 and 0.1 mg·l−1 respectively with a conductivity meter. Nitrites and total iron were measured to the nearest 0.01 mg·l−1 via titration with Merck reagents. During the study period, water depth in the main river channel averaged 421.2 (±210.1 SD) cm and turbidity averaged 40.3 (±28.2) cm. Dissolved oxygen ranged between 0.4 and 4.5 mg/l (9% - 77% saturation). Most other physical and chemical features revealed less variation; mean water temperature during the study was 28.6 (±2.2)˚C, pH averaged 5.4 (±0.6), electric conductivity averaged 99.45 (±3.1) ms/cm, and average total dissolved solid (TDS) was 46.75 (±5.38) mg/l. Nitrite and total iron concentrations averaged 0.002 (±0.0015) mg/l and 1.04 (±0.66) mg/l, respectively. The dominant floating macrophytes on the Sô River are Eicchornia crassipes, a species that invaded the system about 25 years ago. Other common aquatic plants were Pistia stratiotes (Aracea) and Ipomea aquatica (Convolvulaceae). Palms (Elaies guinensis) were the dominant riparian trees.
Lake Hlan is located near Kpomey village (Sehoue City) about 80 km from the Atlantic coast. Locally known as “the Bonytongue Lake”, Lake Hlan receives comparatively low exploitation owing to local enforcement of traditional fishing regulations. Lake Hlan has a lower average depth (250.3 ± 128.5 cm) than the study reach on the Sô River. The annual flood was very low in Lake Hlan during 2002 (maximum water depth = 340 cm), but occurred normally during 2003 (maximum water depth = 670 cm). Dissolved oxygen ranged from 0.1 mg/l (25.47% of saturation) to 4.8 mg/l (79%). Water transparency averaged 88.1 (±25.2) cm, mean water temperature was 27.60 (±1.80)˚C, mean pH was 5.3 (±0.20), average conductivity was 97.0 (±5.5) ms/cm, and mean total dissolved solid (TDS) was 47.0 (± 2.85) mg/l. Nitrites and total iron concentration were low and averaged 0.002 (±0.001) mg/l and 0.67 (±0.26) mg/l, respectively. Floating grasses, such as Cyperus difformis (Cyperaceae), cover large areas of the lake and hinder fishing activities. Wind-driven movement of grass mats sometimes damages fishing gear. These grasses also provide habitat for many aquatic organisms, including small fishes. Water hyacinth is present in Lake Hlan, but at low biomass compared to most areas of the river channel. Other common floating macrophytes in Lake Hlan were Pistia stratiotes, Azolla africana, Nymphaea lotus and N. maculatus (Nymphaeacea), Eicchornia crassipes, Echinochloa pyramides (Poacee). Submerged plants included Ceratophyllum demersum (Ceratophyllaceae) and Utricularia inflexa (Lantibulariaceae).
2.2. Fish Sample Collection
Bonytongue specimens were collected monthly from both aquatic vegetation and open water at four sites in the Sô River and Lake Hlan during eighteen (18) months. At each site, fish individuals were captured with two fishing gears in order to obtain representative samples of all size classes in the local population. In the Sô River, surveys were conducted along a longitudinal reach. Patches of vegetation were encircled with a net (2-m high, 10-mm mesh), and vegetation and fish were removed. Open water areas of the river were sampled with a cast net (50 - 80 mm mesh). In Lake Hlan, fish were collected with traps (80 mm long; 50 mm opening), gill nets (20 m × 2 m, 60-mm bar mesh), and hooks. Local fishermen were enlisted to set traps in vegetation close to the openings of active nests. All captured size classes were retained for analysis. Adult size classes were rare at Sô River survey sites, and monthly sampling effort was continued until collection effort yielded samples that reflected population structure.
Each specimen of Heterotis was measured for standard (SL) and total length (TL) to the nearest 0.1 mm with a graduated measuring board and weighed to the nearest 0.1 g with an electronic balance. Specimens were dissected, and each alimentary canal was removed and its length was measured as the distance from the distal end of one of the two pyloric caecae to the anus (Moreau; 1982). Stomachs were preserved in 5% formalin and transported to the laboratory of the Department of Zoology & Genetics of the University of Abomey-Calavi. Preserved stomachs were removed from the formalin and placed in 75% ethanol for preservation.
2.3. Stomach Content Analysis
The ethanol-preserved stomachs were opened and all preys were removed and spread on a glass slide for examination under a dissecting microscope. Water was added to facilitate separation of small items. A sub-sample of the contents was examined under a compound microscope to identify phytoplankton. Food items were identified to the lowest possibly taxonomic level using Needham and Needham (1962) [21] for aquatic invertebrates, zooplankton, and phytoplankton. After identification, food items were separated and blotted on a paper towel to remove excess moisture. The volume of each food category was estimated by water displacement using an appropriately sized graduated cylinder. Volumes of small items (<0.002 ml) were estimated by spreading the material on a glass slide then visually estimating the collective volume by comparison with a sample of known volume
[22].
2.4. Data Analysis
Food items ingested by the different sizes classes of H. niloticus were aggregated by category, and volumetric proportions of these new aggregate categories were summed.
Volumetric proportions of each food item ingested by H. niloticus were computed following the formula:

where pi is the proportion of food item i in the diet, n is the number of stomachs, vi is the volume of food item i in a single stomach, Vt is the total volume of food ingested by n stomachs (in this study, n = 1461 stomachs).
Diet breadth was calculated following Simpson’s (1949) diet breadth formula (Krebs, 1989) [23,24]:

where pi is the proportion of food item i in the diet, and n is the total number of food items in the diet. B ranges from 1, when only one resource is used, to n, when all resources are consumed in equal proportions. Seasonal and size differences in volumetric proportions and values of indices were compared with analysis of variances (ANOVA) using SPSS software package [25]. Ecomorphological relationships were examined using linear regression analysis between standard length/gut length and the main food items and between standard length/gut length and the diet breadth.
3. RESULTS
3.1. Prey Ingested
In both Lake Hlan and Sô River, H. niloticus consumed a variety of benthic and pelagic prey resources dominated by detritus and sand particles, hard seeds, aquatic insects, microcrustacea and mollusks (Tables 1-6). Aquatic insects were mostly immature stages of Diptera (Chironomidae, Ceratopogonidae, Syrphidae, Tipulidae, Empididae), Ephemeroptera, Coleoptera (Dryopidae, Cybister, Helodidae, Thermonectus), Hemiptera, Odonata, Heteroptera, and Plecoptera. Among aquatic insects, chironomid larvae dominated diets in both Lake Hlan and Sô River with volumetric proportions varying from 5.98% to 39.57% (mean = 17.15%), and from 0% to 38.94% (mean = 21.08%), respectively. Ephemeroptera (means = 4.80% in Lake Hlan vs 0.05% in Sô River) were more common in diets of Lake Hlan fish, whereas Coleoptera (means = 1.67% in Lake Hlan vs 7.56% in Sô River) were more abundant in Sô river fish diets. Microcrustacea were mostly Cladoceran (mainly Daphnidae), Ostracoda (Cypridopsis), Copepoda (mainly Cyclops and Diaptomus), Amphipoda, and Eubranchipoda. In general cladocerans were the most important microcrustaceans in both habitats. Water mites (Hydracharidae) generally were consumed in low proportions, but were common in diets of subadults in Lake Hlan. Gastropod mollusks (Limnidae, Planorbiidae, Hydrobiidae, Physidae) were more abundant in diets of fish from Sô River. Minor diet items were plant tissues (including flowers and fruits), terrestrial insects (Coleoptera, Hymenoptera, Megaloptera), chitin fragments, nematode worms, invertebrate eggs, Rotifera

Table 1. Matrix of food categories consumed by size class of Heterotis niloticus captured in Lake Hlan during the wet season.

Table 2. Matrix of food categories consumed by size class of Heterotis niloticus captured in Lake Hlan during the high-water season.

Table 3. Matrix of food categories consumed by size class of Heterotis niloticus captured in Lake Hlan during the dry season.
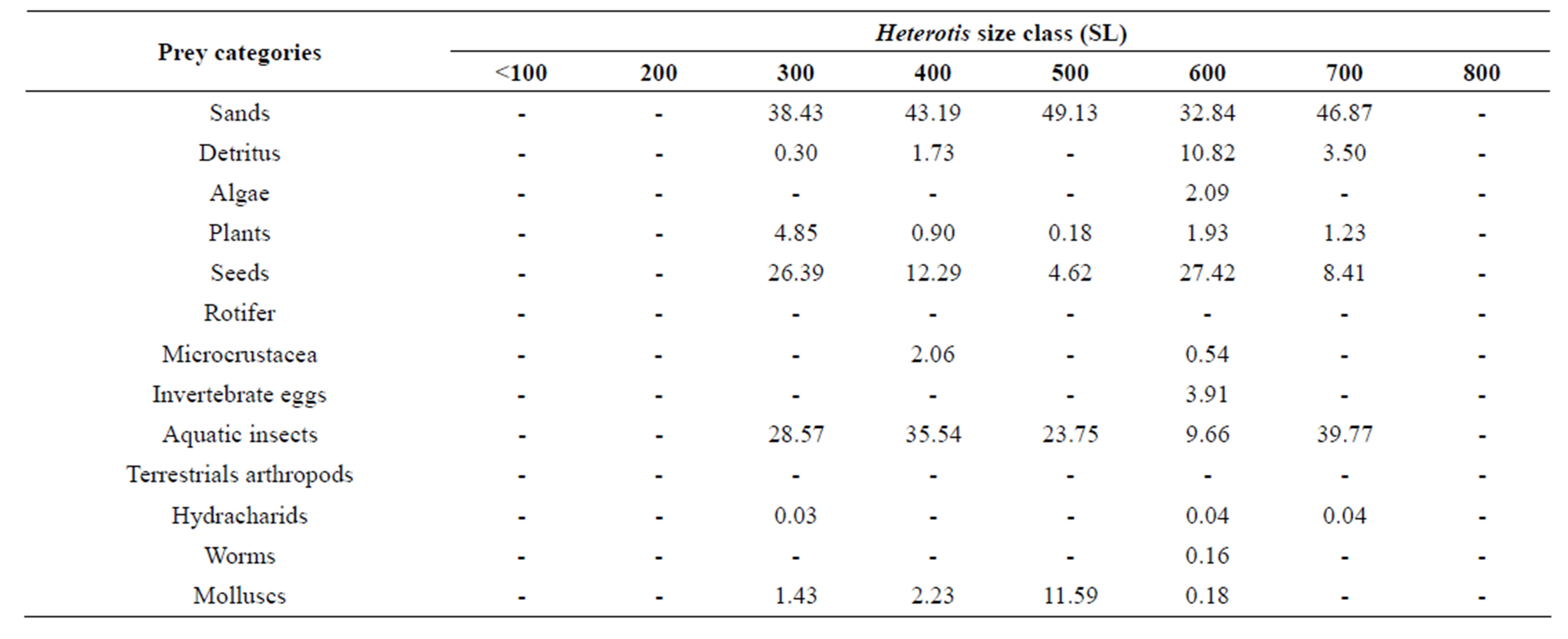
Table 4. Matrix of food categories consumed by size class of Heterotis niloticus captured in the Sô river during the wet season.

Table 5. Matrix of food categories consumed by size class of Heterotis niloticus captured in the Sô river during the high-water season.

Table 6. Matrix of food categories consumed by size class of Heterotis niloticus captured in the Sô river during the dry season.
(Testudinella, Asplanchna, Chromogaster, Brachionus, Euchlanis, Keratella), and various algae taxa such as diatoms (Raphoneis, Pleurosigma, Synedra, Nitzschia, Gomphonema, Melosira, Tabellaria, Asterinella, Navicula), cyanobacteria (Polycistis, Protococcus, Phormidium, Coelosphaerium, Nostoc, Oscillatoria, Merismopodia), green algae (Rhizodonium, Botyoccocus, Ulothrix sp, Richterella, Spirogyra, Coelastrum) and desmids (Gonatozygon, Closterium).
One-way ANOVA revealed significant between-habitat differences in consumed volumetric proportions of some of the dominant food resources. Among aquatic invertebrates, significant differences were found between proportions consumed in the two habitats for Odonata niads (P < 0.01), item that was more common in diets of fish individuals from Lake Hlan, as well as Coleoptera (P <
0.05) and mollusks (P < 0.05) groups that were consumed in greater proportions by individuals from the Sô River. Most of the Coleoptera in stomachs were adults. Volumetric proportions of aggregated microcrustaceans (Ostracoda, Cladocera, Copepoda, Eubranchipoda, Amphipoda) consumed by Heterotis differed in diets of fishes from the two habitats (P < 0.05), with higher proportions associated with individuals from Lake Hlan. Proportional consumption of sand and detritus, seeds, and aggregated aquatic insects did not differ significantly (P ≥ 0.05) between the two habitats. With regard to seasons, in Lake Hlan, proportional consumption of aquatic insects was greater during the high-water season and those of sand & detritus and microcrustacea was greater during the wet season (Tables 1 and 2). Also, in Lake Hlan, proportional consumption of seeds tended to be greater during the dry season (Table 3). In the Sô River, proportional consumption of sand and detritus was greater during the wet season and proportional consumption of seeds was greater during the high-water season (Tables 4 and 5). Also, in the Sô River, the volumetric proportion of aquatic insects ingested by bonytongues was greater during the dry season when inundated plain is shrinking and aquatic insects become more concentrated and more available for Heterotis (Table 6).
3.2. Spatial and Seasonal Variation of Diet Breadth
In spite of the significant differences (P < 0.05) in microcrustacea consumption between Heterotis populations from Lake Hlan and Sô River, which was not the case for detritus & sand, seeds, and aquatic insects, these two habitats failed to show any significant (P < 0.05) differences in the diet breadth. Mean diet breadth for Lake Hlan and Sô River was 3.71 (range = 2.19 - 4.87) and 3.35 (range = 2.08 - 4.58), respectively (Tables 7 and 8). Likewise, the diet breadths of Heterotis population from both habitats, Lake Hlan and Sô River were not significantly (P ≥ 0.05) associated with seasons. In Lake Hlan, diet breadths varied from 1.67 to 4.18 (mean = 3.37) during the wet season, from 2.19 to 4.38 (mean = 3.62) during the high-water season and from 3.78 to 4.66 (mean = 4.18) during the dry season. In the Sô river, diet breadths varied from 2.59 to 4.58 (mean = 3.27) during the wet season, from 1.61 to 4.38 (mean = 3.01) during the high-water season and from 2.92 to 3.96 (mean =
2.95) during the dry season (Tables 7 and 8).
3.3. Ecomorphological Correlates
To explore the ecomorphological patterns of the trophic structure of Heterotis, the volumetric proportion of each main food resource consumed (detritus, seeds, microcrustacea, aquatic insects) were plotted against the standard length (SL) and the gut length (GL). Logarithm transformation of SL and GL was used to reduce skew in the dataset. In both habitats, the linear regression equations showed that Log (SL) and Log (GL) were positively correlated with the volumetric proportion of detritus and with the volumetric proportion of seeds (Table 9). On the contrary, the standard length (Log SL) and the gut length (Log GL) were negatively correlated with the volumetric proportion of aquatic insects and with the volumetric proportion of microcrustacea (Table 9). However, in Lake Hlan, the coefficients of correlation tended to be higher than those of the Sô River (Table 9).
In Lake Hlan, the scatter plot and the linear regression equations (Figures 2 and 3) showed that the diet breadth was positively correlated (P < 0.05) with the standard length (r = 0.81) and the gut length (r = 0.82). On the contrary, in the Sô River, the diet breadth was negatively correlated (but not significant) with the standard length (r = −0.39) and the gut length (r = −0.37) (Figures 2 and 3). As results, the computed coefficient of correlations indicated that DB in Lake Hlan exhibited similar trends with SL (r = 0.81) and GL (r = 0.82). The same pattern was recorded in Sô River with r = −0.39 and r = −0.37 for the regression of DB with SL and GL, respectively.

Table 7. Diet breadth (DB) by size class of Heterotis niloticus captured in Lake Hlan.

Table 8. Diet breadth (DB) by size class of Heterotis niloticus captured in the Sô River.

Table 9. Matrix of coefficient of correlation (r), slope and intercept for the regression between the volumetric proportion of the main foods resources (detritus, seeds, microcrustacea, aquatic insects) and the morphological features (standard length, gut length) of Heterotis niloticus captured in lake Hlan and in the Sô River. Logarithmic transformation of standard length (SL) and gut length (GL) was considered for the regression. N = number of SL size classes.

Figure 2. Relationships between diet breadth (DB) and standard length (SL) of Heterotis niloticus captured in Lake Hlan. DB is positively correlated with Log (SL). Regression equation is: DB = 2.33*Log (SL) - 2.15; r = 0.81; N = 8.

Figure 3. Relationships between diet breadth (DB) and gut length (GL) of Heterotis niloticus collected in Lake Hlan. DB is positively correlated with Log (GL). Regression equation is: DB = 2.56*Log (GL) - 2.97; r2 = 0.82; N = 8.
4. DISCUSSIONS
4.1. Diet Breadth Trends
Because Lake Hlan is a relatively more stable aquatic ecosystem with the presence and the persistence of swamp habitats throughout all the seasons, facilitating permanent colonization and availability of food resources [13], we expect to obtain higher diet breadth in this habitat compared to Sô River. The Sô river, a less stable habitat, although periodically flooded (3 - 4 months) should exhibit a relatively low food resource availability during much time of the year (dry season: 8 - 9 months) and consequently Heterotis population from the Sô river should exhibit a low diet breadth. This was not the case. Mean diet breadths were nearly the same regardless of habitats. This result suggest that, probably, when a main food resource (detritus, seeds, microcrustacea, aquatic insects) availability is low in an habitat (e.g. zooplankton in the Sô River, mollusks in Lake Hlan), Heterotis adjust his foraging efficiency on the available resources to maintain the diet breadth at a top level in order to satisfy their food and nutritional needs.
In term of biodiversity conservation, this trophic pattern is an advantage for species survival and conservation in natural habitats, and may explain why Heterotis is widely distributed in the numerous freshwater systems of Benin. In captivity (aquaculture), the broader diet breadth and this trophic adaptation of Heterotis may facilitate the efficient exploitation of the different levels (detritus, zooplankton, insects) of the trophic chain in the fish ponds [17,18].
In both habitats, diet breadth was not associated with season. However, in the Sô River, the relative low diet breadth of the dry season compared to that of the wet and high water seasons is probably due to the low food resource availability during the dry season when the river is not flooded and is reduced to the main channel. In Lake Hlan, the progressive increase of the diet breadth from wet to dry season is probably due to a progressive colonization (multiplication) of food resources which have become concentrated and more available during the dry season, as water volume is depleted.
The lack of association of the diet breadth with habitats on one hand, and with seasons on the second, suggests that, despite the differences in food resources availability between habitats or throughout seasons, Heterotis manage to adjust his diet (volumetric proportion of food items ingested) in order to maintain nearly the same diet breadth. This prey consumption adjustment and foraging strategy are possible because of Heterotis omnivore diet habit and its specialized morphological structure [7].
4.2. Ecomorphological Patterns
In both habitats, the positive correlation of both standard length Log (SL) and gut length Log (GL) with the volumetric proportions of detritus and seeds suggests that the consumption of these two food resources increased with the size of Heterotis and the development of the digestive tract (Danson-Ofori, 1992; Koen Alonso, 2002) [26,27]. At the early age (<100 mm), the digestive tract of Heterotis is less developed to digest detritus and seeds with hard coats. As the development of the digestive system is initiated with a functional gizzard, Heterotis begin to progressively ingest detritus and seeds. Indeed, the digestive apparatus of Heterotis comprises a gizzard, a thick – walled muscular chamber that facilitates the digestion of refractory organic matter such as plants detritus, hard seeds, carapace and shell (Bowen, 1983; Adite et al., 2005) [13,28]. Likewise, the negative correlation of both standard length Log (SL) and gut length Log (GL) with the volumetric proportions of aquatic insects and with those of microcrustacea suggests that the consumption of this two food categories decreased as the sizes and the gut length of Heterotis increased. In general, small fish individuals with less developed digestive system forage heavily on live food such as microcrustacea, insect larva and nymphs (Garcia-Berthou, 1999; Garcia-Berthou and Moreno-Amich, 2000) [29,30]. However, as digestive tract becomes well developed, Heterotis takes advantage to other available resources such as detritus and seeds to rapidly satisfy his high protein and energetic requirements (Steingrimsson and Gislason, 2002) [31]. The low negative correlations of standard length and gut length with microcrustacea compared to those with aquatic insects were probably due to the presence of gill rakers (42 - 94 rakers on the first branchial arch) (Moreau, 1982) during the whole life of Heterotis, which facilitate sieving of zooplankton and other microcrustacea regardless of age or size (Adite et al., 2006) [8,32]. For example, a 605 mm specimen, collected from Lake Hlan have consumed 100% (volume = 3 ml) of microcrustacea, mainly cladoceran and copepod.
In Lake Hlan, mean diet breadth tended to increase with size (r = 0.81) and gut length (r = 0.82) indicating that bonytongues ingest a broader range of food resources as they grow (Winemiller, 1989) [22]. This patterns was not apparent for Sô River (Figures 2 and 3) because of the low sample size for the 700 mm SL (N = 6) size class and for the 800 mm SL (N = 1) size class, and probably led to an underestimate of diet breadth for these size classes in the Sô River.
4.3. Trophic Plasticity Behavior: Implications for Species Conservation and Aquaculture Development
When examining the volumetric proportion of the main food items (detritus, seeds, microcrustacea, aquatic insects) consumed by Heterotis in the two habitats, the microcrustacea ingested in the Sô River was significantly (P < 0.05) lower compared to that consumed by the population of Lake Hlan. The mean volumetric proportions of microcrustacea in Lake Hlan and Sô River were 13.77% (range: 4.41% - 27.07% for 600 mm SL and 200 mm SL size classes, respectively) and 2.63% (range: 0% - 6.32% for 800 mm SL and 500 mm SL size classes, respectively), respectively. This high gap in the consumption of microcrustacea between the two populations was probably due to the low availability of this food resource in the Sô River and may result from the differences in habitat characteristics: the Sô River is a lotic environment (running water) which undergoes a high disturbance during the flooding season creating a high turbidity. This disturbance may inhibit photosynthesis and consequently may reduce primary production (phytoplankton) and zooplankton proliferation.
In the Sô River, this low ingestion of microcrustacea was associated with a relatively high consumption of mollusks compared to Lake Hlan. Mean volumetric proportions of mollusks consumed in the Sô river and lake Hlan were 4.91% (range: 0% - 11.59%) and 0.73% (range: 0% - 1.65% ), respectively. Therefore, the zooplanktinovore trend of Heterotis diet habit observed in Lake Hlan was not apparent in the Sô River, probably because of the low availability of zooplankton in this habitat. Likewise, the relatively high consumption of mollusks in the Sô River was not apparent in Lake Hlan where this food item is likely less available.
As a result, this foraging pattern suggests a degree of trophic plasticity in Heterotis, which may shift his trophic structure according to resource availability in the habitat [13,33]. This trophic plasticity behavior is favored by the specialized morphological structure of Heterotis, mainly the presence of a relatively high number of gill rakers (42 - 94 rakers on the first branchial arch) [7,8] during its whole life, allowing sieving of zooplankton and other microcrustacea, and the presence of the gizzard that facilitates the digestion of refractory organic matter such as mollusks with hard carapace [13,28]. Also, the omnivorous diet habit and the relatively high diet breadth resulting from this specialized morphological structure favored such trend of trophic plasticity in Heterotis.
In the Sô River, despite the relatively high availability of both seeds and aquatic insects resulting from their periodic invasion caused by the intrusion of flood water in the relatively dense adjacent vegetation, Heterotis did wider its consumption towards mollusks, source of animal protein. These foraging behavior suggest that this species take advantage to the available preys to probably maintain a balance diet and to satisfy energetic and protein requirements for rapid growth.
The trophic plasticity behavior, presently evidenced in Heterotis, is common in migratory species and in fish species inhabiting unstable habitats. For example, in lake Sibaya (South Africa), Bowen et al. (1982) [34] have reported a similar trend of trophic plasticity with Tilapia mossambicca (Cichlidae) whose diet changed from benthic detrital aggregate to periphyton while moving from a deep offshore water to shallow (0.5 m) littoral areas.
In addition to the broader diet breadths, the trophic plasticity behavior depicted is an advantage for the survival, the conservation and the aquaculture development of Heterotis because enable the species not only to colonize and to adapt to unstable and changing aquatic habitats, but also to invade and to well establish in many habitats with different features (physical characteristics; food availability, niche breadths and niche overlaps), such as freshwater lakes, swamps, inundated plains, streams, rivers and fish ponds capable of varying water level in order to stimulate gonad maturation and to insure reproduction success [32,35].
5. CONCLUSION
Currently, among the African freshwater fishes, H. niloticus is considered as an important key species under genetic, bio-ecology, fishery and aquaculture researches because of its large size, high growth rate, the omnivore food habit and its overexploitation in the highly degrading aquatic environment. The output of the present investigation on the diet composition, the diet breadth and the trophic plasticity gives valuable information for the conservation, the sustainable fisheries management and the farming development of this highly economic and commercial species.
6. ACKNOWLEDGEMENTS
The Section of Ecology and Evolutionary Biology, Department of Wildlife and Fisheries Sciences of Texas A&M University provided financial assistance and logistic support during data analysis. We are sincerely grateful to Dr Kirk O. Winemiller for his assistance in all phases of the research project. Also, we are grateful to the fishermen of Lake Hlan and the Sô River for their hospitality and assistance during sampling.
REFERENCES
- Greenwood, P.H., Rosen, D.E., Weitzman, S.H. and Myers, G.S. (1966) Phyletic studies of Teleostean fishes with a provisional classification of living forms. Bulletin American Museum of Natural History, 131, 339-456.
- Scott, D.B.C. (1974) The reproductive cycle of Mormyrus kannume Forsk. (Osteoglossomorpha, Mormyriformes) in Lake Victoria, Uganda. Journal of Fish Biology, 6, 447- 454. http://dx.doi.org/10.1111/j.1095-8649.1974.tb04560.x
- Li, G.-Q. and Wilson, M.V.H. (1996) Phylogeny of osteoglossomorpha. In: Stiassy, M.I.J., Parenti, L.R. and Johson, G.D., Eds., Interrelationships of Fishes, Academic Press, New York, 163-174.
- Holden, M. and Reed, W. (1972) West African freshwater fish. Longman Group Ltd., London.
- Greenwood, P.H. (1973) Interrelationships of the Osteoglossomorphs. In: Greenwood, P.H., Miles, R.S. and Patterson, C., Eds., Interrelationships of Fishes, Academic Press, London, 307-320.
- Moreau, J. (1974) Premières, observations écologiques sur la reproduction d’ Heterotis niloticus (Osteoglossidae). Annales Hydrobiologie, 5, 1-13.
- Aubenton, F. (1955) Etude de l’appareil branchiospinal et de l’organe suprabranchial de Heterotis niloticus (Cuv.). Bulletin de l’Institut Français d’Afrique Noire, 17, 1179- 1201.
- Moreau, J. (1982) Exposé synoptique des données biologiques sur Heterotis niloticus (Cuvier, 1929). Food and Agriculture Organization Synopsis de pêches, 131, 1-45.
- Leveque, C., Paugy, D. and Teugels, G.G. (1990) Faunes des poissons d’eaux douces et saumâtres de l’Afrique de l’Ouest. Tome 1, Editions ORSTOM/MDRAC, Paris.
- Depierre, D. and Vivien, J. (1977) Une réussite du service forestier de Cameroun: l’introduction de Heterotis niloticus dans le Nyong. Bois Forêt Tropiques, 179, 59-66.
- Hurtado, L.A., Carrera, E., Adite, A. and Winemiller, K.O. (2013) Genetic differentiation of a primitive teleost, the African bonytongue Heterotis niloticus, among river basins and within a floodplain river system in Benin, West Africa. Journal of Fish Biology, 83, 682-690. http://dx.doi.org/10.1111/jfb.12198
- Van Thielen, R., Hounkpe, C., Agon, G. and Dagba, L. (1987) Guide de détermination des Poissons et Crustacés des Lagunes et Lacs du Bas-Bénin. Direction des Pêches, Cotonou, Bénin.
- Adite, A., Winemiller, K.O. and Fiogbe, E.D. (2005) Ontogenetic, seasonal, and spatial variation in the diet of Heterotis niloticus (Osteoglossiformes: Osteoglossidae) in the Sô River and Lake Hlan, Benin, West Africa. Environmental Biology of Fishes, 73, 367-378. http://dx.doi.org/10.1007/s10641-004-5563-9
- Fagade, S.O. and Olaniyan, C.I.O. (1973) The foods and feeding interrelationships of the fishes in the Lagos lagoon. Journal of Fish Biology, 5, 205-225. http://dx.doi.org/10.1111/j.1095-8649.1973.tb04449.x
- Lowe McConnell, R.H. (1975) Fish communities in the tropical freshwaters. Longman, London.
- Lowe McConnell, R.H. (1987) Ecological studies in the in Tropical Fish communities. Cambridge University Press, Cambridge. http://dx.doi.org/10.1017/CBO9780511721892
- Welcomme, R.L. (1979) Fisheries ecology of floodplains rivers. Longman, New York.
- Lauzanne, L. (1976) Régimes alimentaires et relations trophiques des poissons du lac Tchad. Cahiers ORSTOM, Sér. Hydrobiologie, 10, 267-310.
- Hickley, P. and Bailey, R.G. (1987) Foods and feeding relationships of the fish in the Sudd swamps. Journal of Fish Biology, 30, 147-159. http://dx.doi.org/10.1111/j.1095-8649.1987.tb05741.x
- ASCENA (2003) Données sur la pluviométrie des années 2002 et 2003. Agence pour la sécurité de la navigation Aérienne, Service de la Méréorologie Nationale Cotonou.
- Needham, G.J. and Needham, P.R. (1962) A guide to the study of fresh-water Biology. Holden Day, San Franscisco, California.
- Winemiller, K.O. (1989). Ontogenetic diet shifts and resource partitioning among piscivorous fishes in the Venezuelan llanos. Environmental Biology of Fishes, 26, 177- 199. http://dx.doi.org/10.1007/BF00004815
- Simpson, E.H. (1949) Measurement of diversity. Nature, 163, 688. http://dx.doi.org/10.1038/163688a0
- Krebs, C.J. (1989) Ecological methodology. Harper & Row Publisher, New York.
- Morgan, G.A., Grieggo, O.V. and Gloekner, G.W. (2001) SPSS for windows: An introduction to use and interpretation in research. Lawrence Erlbaum Associates, Publishers, Mahwah.
- Danson-Ofori, P. (1992) Ecology of some species of catfish Synodontis (Pisces: Mochocidae) in the Kpong Headpond in Ghana. Environmental Biology of Fishes, 35, 49- 61. http://dx.doi.org/10.1007/BF00001157
- Koen Alonso, M., Crespo, E.A., Garcia, N.A., Pedraza, S.N., Mariotti, P.A. and Mora, N.J. (2002) Fishery and ontogenetic driven changes in the diets of the spiny dogfish, Squalus acanthias, in Patagonian waters, Argentina. Environmental Biology of Fishes, 63, 193-202.
- Bowen, S.H. (1983) Detritivory in the neotropical fish communities. Environmental Biology of Fishes, 9, 137- 144. http://dx.doi.org/10.1007/BF00690858
- Garcia-Berthou, E. (1999) Food of introduced mosquitofish: Ontogenetics diet shift and prey selection. Journal of Fish Biology, 55, 135-147. http://dx.doi.org/10.1111/j.1095-8649.1999.tb00663.x
- Garcia-Berthou, E. and Moreno-Amich, R. (2000) Food of introduced pumpkinseed sunfish: Ontogenetic diet shift and seasonal variation. Journal of Fish Biology, 57, 29- 40. http://dx.doi.org/10.1111/j.1095-8649.2000.tb00773.x
- Steingrimsson, S.O. and Gislason, G.M. (2002) Body size, diet and growth of landlocked brown trout, Salmon trutta, in the subarctic River Laxa, North-East Iceland. Environmental Biology of Fishes, 63, 417-426. http://dx.doi.org/10.1023/A:1014976612970
- Adite, A., Winemiller, K.O. and Fiogbe, E.D. (2006) Population structure and reproduction of the African bonytongue Heterotis niloticus in the Sô River-floodplain system (West Africa): Implications for management. Ecology of Freshwater Fishes, 15, 30-39. http://dx.doi.org/10.1111/j.1600-0633.2005.00119.x
- Winemiller, K.O. and Kelso-Winemiller, L.C. (2003) Food habits of tilapiine cichlids of the Upper Zambezi River and floodplains during the descending phase of the hydrological cycle. Journal of Fish Biology, 63, 120-128. http://dx.doi.org/10.1046/j.1095-8649.2003.00134.x
- Bowen, S.H. and Allanson, B.R. (1982) Behavioral and trophic plasticity of juvenile Tilapia mossambica in utilization of the unstable littoral habitat. Environmental Biology of Fishes, 7, 354-362. http://dx.doi.org/10.1007/BF00005570
- Pianka, E.R. (1994) Evolutionary ecology. 5th Edition, Harper Collins College Publishers, New York.

