World Journal of Vaccines
Vol.3 No.1(2013), Article ID:27965,9 pages DOI:10.4236/wjv.2013.31001
MontanideTM Gel01 ST Adjuvant Enhances PRRS Modified Live Vaccine Efficacy by Regulating Porcine Humoral and Cellular Immune Responses
![]()
1Department of Anatomy and Physiology, College of Veterinary Medicine, Kansas State University, Manhattan, USA; 2Department of Diagnostic Medicine and Pathobiology, College of Veterinary Medicine, Kansas State University, Manhattan, USA; 3Virus and Prion Research Unit, National Animal Disease Center, United States Department of Agriculture, Ames, USA.
Email: *jshi@vet.k-state.edu
Received November 17th, 2012; revised December 18th, 2012; accepted December 28th, 2012
Keywords: PRRSV; MontanideTM Gel01 ST; IFN-γ; Adjuvant; IL-10; Immune Response; Vaccine
ABSTRACT
Porcine reproductive and respiratory syndrome (PRRS) is a devastating disease caused by the PRRS virus. The MontanideTM class of flexible polymeric adjuvants has recently been shown to enhance protective immunity against PRRSV infection in piglets when used in combination with PRRS modified live vaccines (MLV). In this study, we explored the efficacy and immunological mechanisms of protection of MontanideTM Gel01 ST (Gel01) adjuvanted modified live PRRSV vaccine in pigs challenged with two genetically distinct strains of PRRSV. Gel01-MLV reduced lymph node pathology scores in pigs challenged with VR-2332 (parental strain of MLV vaccine) but not that in pigs challenged with MN184A (heterologous strain), when compared to that in pigs vaccinated with un-adjuvanted MLV. Pigs vaccinated with Gel01-MLV had higher levels of PRRS-specific antibodies, as measured by IDEXX ELISA and virus neutralizing antibodies, after vaccination and VR-2332 challenge. In addition, pigs vaccinated with Gel01-MLV had decreased levels of IFN-γ, IL-10, and T-regulatory lymphocytes in the blood as compared to that in pigs vaccinated with MLV alone. Interestingly, we found that addition of Gel01 did not change the profile of other T lymphocyte populations after PRRSV challenge. These results demonstrate that the MLV adjuvanted with Gel01 provides enhanced protection against homologous PRRSV infection, possibly by regulating the production of PRRSV-specific antibodies and cytokines involved in the development of T-regulatory cells. Thus, Gel01 ST is a promising adjuvant that can be formulated with PRRSV MLV vaccines to reduce disease severity and tissue damage caused by PRRSV infection in pigs.
1. Introduction
Porcine reproductive and respiratory syndrome (PRRS) is currently one of the most devastating swine diseases worldwide, causing immense economic losses in the swine industry [1]. It was estimated that the US pork industry alone has annual losses of $664 million due to the prevalence of PRRS [2]. The causative agent, PRRS virus (PRRSV), belongs to the family Arteriviridae, order Nidovirales, and causes reproductive failure in sows including still births, mummification, week-born piglets and high pre-weaning mortality [3]. Currently, comercially available PRRS modified live vaccines (MLVs) are widely being used in the US to control PRRSV infection [4]. However, the efficacy of these MLVs is debated due to limited protection against antigenically diverse heterologous PRRS virus isolates [5].
Current formulations of MLV do not contain adjuvants since the multiplication and infectious properties of attenuated live PRRS virus have been shown to induce sufficient protection to PRRSV infection [6]. However, due to the high degree of genetic variation of PRRSV, new strains are quickly emerging that current vaccine formulations may not be able to protect against. Recently, several studies showed that addition of adjuvants, such as Mycobacterium tuberculosis whole cell lysate, to PRRS modified live vaccine (PRRS-MLV) can induce enhanced cross-protective immunity to PRRSV [7]. However, the addition of these experimental adjuvants to comercially available vaccines still requires large-scale trials and certification by USDA before they can be brought to market. By contrast, the MontanideTM class of adjuvants is a well-established brand of vaccine adjuvants, which are already approved in Europe and included in several registered commercial veterinary vaccines for food animals including cattle, poultry, and fish. These MontanideTM adjuvants have been shown to enhance disease protection when combined with diverse types of antigens [8]. Recently, one research group used MontanideTM Gel01, a polymer based adjuvant, as adjuvant for PRRS attenuated live vaccine and found the addition of Gel01 enhanced protection from PRRS in vaccinated animals, even in formulations containing half the dose of the modified live PPRSV [9]. However, they did not evaluate the cross-protection potential of the Gel01 adjuvant or mechanism of increased protection. Therefore, using Gel01 ST as an adjuvant, we evaluated PRRS MLV-induced humoral and cellular immune responses to homologous and heterologous PRRSV challenges and explored whether Gel01-adjuvanted PRRS MLV can provide broader crossprotection to field strains of PRRSV.
2. Materials and Methods
2.1. Cells, Virus and Adjuvant Preparation
MARC-145 cells were maintained in Modified Eagle’s medium (MEM) supplemented with 7% fetal bovine serum (FBS) containing 100U penicillin/ml and 100 ug streptomycin/ml at 37˚C with 5% CO2. Virus stocks were prepared and titrated in MARC-145 cells and stored in aliquots in −80˚C until use. For virus infection and titration, MEM supplemented with 2% FBS was used. Modified live virus vaccine (PRRS-MLV) was purchased from Boehringer Ingelheim Vetmedica Inc. PRRSV VR-2332, the parental strains of MLV, was purchased from ATCC. PRRSV MN184A was a kind gift from Dr. Kay Faaberg in United States Department of Agriculture. MontanideTM Gel01 ST (Gel01) polymeric adjuvant was a kind gift from Dr. Robert Parker (SEPPIC Inc.). A final 10% of Gel01 was added into diluted PRRS modified live vaccine and mixed by manual shaking.
2.2. Pigs, Vaccination and Challenge
Thirty-five conventional large White-Duroc crossbred weaned specific-pathogen free piglets at 3 weeks of age were housed at the Large Animal Research Center (LARC) facility, within Kansas State University. These piglets were confirmed sera-negative for antibodies to PRRSV by ELISA and PRRSV-free in the blood by RT-PCR. Pigs were allowed to acclimate for an additional week before initiation of the experiment. Pigs were immunized intramuscularly on day post-vaccination (DPV) 0 with MEM (placebo) or vaccine (PRRS-MLV, 1 × 106 TCID50/pig) formulated with or without 10% Gel01 adjuvant. After four weeks, the pigs were challenged with either homologous PRRSV VR-2332 (1 × 106 TCID50) or heterologous PRRSV MN184A (5 × 105 TCID50). Pigs were monitored for body weight once a week and clinical signs of infection, including rectal temperature, for 7 days post challenge (DPC).
2.3. Collection of Blood Samples for Analysis
Blood was collected on DPV 0, 7, 14, 21, 28, DPC 7 and 14. Serum was separated from clotted blood and preserved at −20˚C until used in assays. Serum was used for evaluation of viremia, viral titer, serum neutralizing antibody titers, and ELISA antibody titer (HerdCheck Porcine Reproductive and Respiratory Syndrome Antibody test Kit, IDEXX Laboratories) to PRRSV as suggested by the manufacturer. Peripheral blood mononuclear cells (PBMCs) were isolated from a heparinized blood sample by Ficoll-Hypaque gradient centrifugation using Histopaque®-1077 (Sigma-Aldrich, St. Louis, MO). PBMCs were used for ELISpot assay, flow cytometry and realtime PCR analysis.
2.4. Gross Lung and Lymph Node Lesion Analysis
Pigs were humanely euthanized on DPC 14 as approved by Kansas State University Institutional Animal Use and Biosafety Committees. To evaluate lung and lymph node histopathology, slices of lung tissue from each lobe and lymph nodes were formalin-fixed, paraffin embedded, sectioned and stained with hematoxylin and eosin (H&E). Scoring of macroscopic and microscopic lung/lymph node pathology was done in a blinded fashion by two veterinary pathologists in the Kansas State Veterinary Diagnostic Laboratory (KSVDL).
2.5. Virus Neutralizing Antibody Titration
Serum samples were heat inactivated (56˚C, 30min) and serially diluted before the titration. The serial dilutions of serum were mixed with equal volumes of PRRSV VR- 2332 or MN184A, respectively, containing 200 TCID50 of virus. After incubation at 37˚C for 1 h, the mixtures were transferred to MARC-145 monolayers in 96-well plates. After incubation for 72 h at 37˚C in a humidified atmosphere containing 5% CO2, cells were examined for cytopathic effect (CPE). CPE was used to determine the end-point titers that were calculated as the reciprocal of the highest serum dilution to neutralize 200 TCID50 of PRRSV in 50% of the wells.
2.6. ELISpot Assay
Briefly, 5 × 105 PBMCs were plated in enriched RPMI in a 96-well multiscreen plate (Millipore, Billerica, MA) pre-coated overnight with capture IFN-γ mAB (BD Pharmingen, San Diego, CA). PBMCs were restimulated with three different strains of PRRSV at 0.1 TCID50 for 24 h at 37˚C. IFN-γ-secreting cells were detected by biotinylated anti-pig IFN-γ detection antibody and visualized using the immunospot image analyzer (Cellular Technology, Cleveland, OH). Data were presented as the mean number of antigen-specific IFN-γ-secreting cells per 106 PBMCs from duplicate wells of each sample.
2.7. Flow Cytometry Analysis
Flow cytometry analysis was performed to determine different lymphocyte populations based on the cell surface marker phenotype: T-helper cells (CD3+CD4+CD8−); cytotoxic T lymphocyte (CD3+CD4−CD8+); Th/memory cells (CD3+CD4+CD8+), T-regulatory cells (CD4+FoxP3+CD25+) and γδ T cells (CD8+ TcR1N4+). Mouse anti-pig TcR1N4 antibody was purchased from VMRD (Pullman, WA), and the rest of the antibodies used in this study were purchased from BD Biosciences. Immuno-stained cells were acquired using a FACS Caliber (BD Biosciences) flow cytometer. Frequencies of individual lymphocytes were analyzed by 100,000 events using FlowJo software (Tree Star, Inc., OR, USA).
2.8. Analysis of Serum PRRSV Titer
Total RNA was extracted from 100 ul serum using TRIzol® Reagent (Sigma). One-step SyBR Green real-time PCR (Bio-Rad) was performed to evaluate PRRSV ORF7 expression level as previously described [10]. For quantification, total RNA of known TCID50 of viruses were 10-fold serially diluted and were used to generate a standard curve. The virus quantities of unknown samples were determined by linear extrapolation of the Ct value plotted against the standard curve.
2.9. Analysis of IL-10 Cytokine Response
Pig sera collected at necropsy and culture supernatants harvested after in vitro re-stimulation of one million of PBMCs, TBLNs, and lung MNCs were analyzed by ELISA kit (Invitrogen, CA) for secretion of IL-10 cytokine.
2.10. Statistical Analysis
All data were expressed as the mean value of five pigs ± SEM. The differences among each group were determined by the paired t test (Prism5.0, GraphPad Software, San Diego, CA). Differences were considered statisticcally significant when P < 0.05.
3. Results
3.1. Addition of Gel01 Adjuvant to MLV Provided Enhanced Protection against Homologous VR-2332 Challenge but Not Heterologous MN184A PRRSV Challenge in Pigs
Currently, the most effective vaccines for PRRS, includeing MLV, are cell-culture attenuated strains of PRRSV. However, the high incidence of genetic mutation during PRRSV transmission often results in vaccines based on strains of PRRSV isolated twenty years ago, such as MLV, having limited protection to new emerging viral strains. Therefore, there is a growing need to develop new vaccines or significantly improve the ones currently available. Previous studies have shown that the MontanideTM line of adjuvants may be able to improve the protection potential of commercially available PRRSV MLV to emerging field isolates of PRRSV [9]. To determine the cross-protection potential of Gel01, pigs were mock vaccinated or vaccinated with modified live PRRSV vaccine formulated with or without Gel01 adjuvant.
Twenty eight days after vaccination, pigs were challenged with homologous VR-2332 (isolated in 1992) or heterologous MN184A (isolated in 2002) strains of PRRSV. Gel01 was tested to be safe when combined with MLV in our vaccination protocol. We did not observe injection site reactions in any group (data not shown) and pigs vaccinated with MLV adjuvanted with Gel01 had equivalent net body weight gain compared to control challenged pigs (Figure 1).
Clinically, unvaccinated pigs developed typical PRRSV symptoms including slight fever and lethargy after challenge. The mean body temperature of unvaccinated pigs challenged with VR-2332 or MN184A was 0.3˚C or 1.0˚C higher, respectively, compared to At necropsy, 14 days post challenge (DPC), Gel01-MLV vaccinated pigs had slightly lower lung lesion scores, although not statistically significant, compared to MLV vaccinated pigs challenged with VR-2332 (Figure 1). However, lymph node pathology scores were significantly lower in Gel01- MLV pigs than MLV vaccinated pigs with homologous VR-2332 challenge (Figure 1). Interestingly, Gel01 adjuvant addition was unable to reduce MN184A-induced lung lesion and lymph node pathology scores. Protection from disease in vaccinated pigs with or without Gel01 was also associated with a significantly reduced PRRSV titer at DPC14 (Figure 1). Circulating VR-2332 PRRSV was cleared in the blood by DPC14 in both MLV and Gel01-MLV vaccinated groups, and a reduced MN184A PRRSV titer in the blood was observed in both vaccinated groups. However, there was no difference between Gel01-MLV and MLV vaccinated groups for the level of viremia (Figure 1).
 (a)
(a) (b)
(b)
Figure 1. Addition of Gel01 adjuvant to MLV provided enhanced protection against homologous VR-2332 challenge but not heterologous MN184A PRRSV challenge in pigs. (a) The body weight of pigs was monitored weekly for 6 weeks starting on the day of vaccination (DPV 0) and concluding 14 days post PRRSV challenge (14 DPC). Fold body weight gain of each individual pig was calculated by normalizing the weight of the pig on DPV 0 to 1; (b) Lung tissue harvested on 14 DPC was sectioned, stained with H&E, examined, and given an estimated score of 0 to 4 based on the severity interstitial pneumonia; (c) Lymph node sections harvested on 14 DPC were examined and given a score from 1 to 3 according to the amount of hyperplasia; (d) PRRSV-specific viral RNA in serum was detected by real-time PCR on 14 DPC. Data are shown as mean ± SEM for five pigs per group. An asterisk denotes statistically significant (P < 0.05).
Taken together, our results suggest that addition of Gel01 to MLV can enhance protection of homologous but not heterologous PRRSV infection in pigs.
3.2. Pigs Vaccinated with Gel01 Adjuvanted MLV Have Enhanced PRRSV-Specific Antibodies and Virus Neutralizing Antibodies after Homologous PRRSV Challenge
Since pigs vaccinated with Gel01-adjuvanted MLV demonstrated enhanced protection against homologous PRRSV challenge, we next wanted to explore the immunological mechanisms of improved vaccination efficacy. The presence of vaccine-induced PRRSV-specific antibodies has been shown to correlate with the protection against disease [11]. Therefore, serum samples were analyzed for PRRSV specific ELISA antibodies and neutralizing antibodies before and after PRRSV challenge. Pigs vaccinated with Gel01-MLV developed significantly higher IDEXX ELISA antibody titers (indicated by value of S/P) on 21 DPV than the MLV vaccinated or unvaccinated pigs (Figure 2).
After challenge, the ELISA antibody titers were only significantly higher in Gel01-MLV vaccinated pigs than that in MLV vaccinated pigs when challenged with homologous VR-2332 (Figure 2). The presence of VR- 2332 and MN184A PRRSV strain-specific neutralizing antibodies were also assayed in the serum of all groups of pig at 14 DPC. Neutralizing antibody titers to VR- 2332 were higher in Gel01MLV pigs than that in MLV vaccinated pigs when challenged with VR-2332 (Figure 2). But there was no difference in neutralizing antibody titers to MN184A between these two vaccinated groups when pigs were challenged with VR-2332 or MN184A. Therefore, our results suggest that Gel01 adjuvant may be facilitating the production of PRRSV-specific antibodies, including neutralizing antibodies, leading to enhanced protection against VR-2332 challenge.
3.3. Pigs Vaccinated with Gel01 Adjuvanted MLV Have Decreased PRRSV-Specific IFN-γ and IL-10 Cytokines after PRRVS Challenge
Our results thus far show that the addition of Gel01 adjuvant to the MLV PRRSV vaccine acts to increase the humoral immune response in pigs challenged with homologous PRRSV. In addition to antibody responses, cytokines expression profiles and cell-based immune responses are involved in the resolution of PRRSV infections [12]. In order to determine whether cellular immune responses were also enhanced by Gel01 adjuvant, PBMCs were isolated from blood samples in each group at 14 DPC. We found that the Gel01-MLV group of pigs challenged with VR-2332, as compared to MLV group, developed a lower frequency of IFN-γ-secreting cells when re-stimulated with the homologous virus (Figure 3).
Additionally, the secretion of immunosupressive cytokine IL-10 by PBMCs was also reduced in the Gel01- MLV vaccinated pigs, but not in MLV pigs, challenged with VR-2332 or MN184A (Figure 3).
To further confirm the decreased IL-10 cytokine expression induced by the addition of the Gel01 adjuvant, serum IL-10 and IL-10 secreted by lung MNCs were also analyzed. As shown in Figure 3, reduced IL-10 cytokine secretion was also found in the serum, but not in the supernatant of lung MNC, of the Gel01-MLV vaccinated pigs challenged with homologous VR-2332 but not het erologous MN184A virus. Therefore, these results suggest that Gel01 adjuvant may increase MLV-mediated protection against homologous PRRSV infection using a mechanism that involves decreased production of circu-
 (a)
(a) (b)
(b)
Figure 2. (a) Pigs vaccinated with Gel01 adjuvanted MLV have enhanced PRRSV-specific antibodies and virus neutralizing antibodies after homologous PRRSV challenge; (b) PRRSV-specific IDEXX ELISA S/P ratio in each group after vaccination and challenge. The ELISA threshold for positive sera was set at a sample to positive (s/p) ratio of 0.4 according manufacturer’s instructions; (c) Individual serum samples collected on 14 DPC were titrated in MARC-145 cells. Anti-PRRSV neutralizing Ab titers were determined as the highest serum dilution that could inhibit CPE. Data are shown as mean ± SEM for five pigs per group. An asterisk denotes statistically significant (P < 0.05).
 (a)
(a) (b)
(b)
Figure 3. Pigs vaccinated with Gel01 adjuvanted MLV have decreased PRRSV-specific IFN-γ and IL-10 cytokines after PRRVS challenge. (a) PBMCs collected from pigs at 14 DPC were re-stimulated with VR-2332 or MN184a for 24 hrs. IFN- γ-secreting cells were then analyzed by the ELISpot assay; (b)-(d) Blood samples were collected from pigs at 14 DPC. Serum and PBMC supernatants were then subjected to ELISA analysis for IL-10 secretion. Lung MNCs were also collected at necropsy (14 DPC), re-stimulated with PRRSV, and subjected to IL-10 detection by ELISA. Data are shown as mean ± SEM for five pigs per group. An asterisk denotes statistically significant (P < 0.05).
lating IFN-γ and IL-10.
3.4. Pigs Vaccinated with Gel01 Adjuvanted MLV Had Reduced T-Regulatory Cell Populations after PRRSV Challenge
Finally, since the immune response and cytokine expression patterns are modulated by different T cell sub-populations, the phenotype and frequency of various lymphoid immune cells in pigs were also analyzed by flow cytometry. The frequency of different immune cells at 14 DPC are shown in Table 1. Interestingly, we found a significant decrease of the T-regulatory cell population in TBLNs and lung MNCs in Gel01-MLV vaccinated pigs compared to the MLV vaccinated pigs after both VR- 2332 and MN184A challenge (Figure 4).
We did not observe any significant differences among total T cell population, T-helper cells, cytotoxic T cellsTable 1. Frequency of T cell subpopulations in pigs after challenge with PRRSV. PBMCs were isolated from blood collected from pigs at necropsy (14 DPC), and T cell subsets were counted by flow cytometry according to their phenotypes. Each number is expressed as the average percent of total PBMCs from five pigs ± SEM. An asterisk indicates a statistically significant difference between unvaccinated or MLV vaccinated and Gel01-MLV pigs.

(a)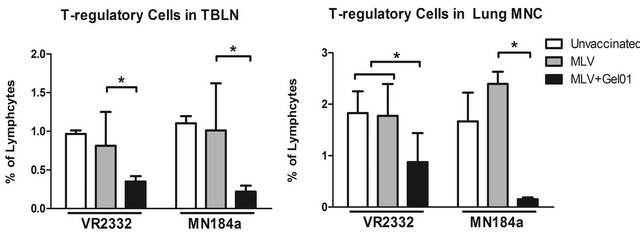 (b)
(b)
Figure 4. Pigs vaccinated with Gel01 adjuvanted MLV had reduced T-regulatory cell populations after PRRSV challenge ((a) and (b)) TBLN cells and lung MNCs were isolated from pigs at necropsy (14 DPC). Total cell population was gated as CD4+ cells, T-regulatory cells were further gated on CD25+ and FoxP3+ expression. Each bar is an average percent of T-regulatory cells from five pigs ± SEM. An asterisk indicates a statistically significant difference (P < 0.05).
Th/memory cells, or γδ T cells (Table1). Taken together, our results suggest that, when combined with PRRSV MLV, Gel01 adjuvant can enhance the protection against homologous PRRSV infection by regulating the development of T regulatory cells.
4. Discussion
Modified live vaccines are widely used in veterinary medicine, as well as in human medicine, to control many infectious diseases in a wide variety of hosts [13].
Currently, almost all commercially available PRRSV vaccines are modified live vaccines based on cell cultureattenuated strains of PRRS virus. In general, MLV provides decent protection against homologous virus infection; however the antigenic disparity of rapidly emerging field isolates leads to partial protection against heterologous viruses [1].
Furthermore, newly emerging isolates are more virulent then parental strains and more prevalent in swine farms across the world, leading to devastating economic losses [2]. Thus, there is a growing need to improve the current PRRSV vaccination practices in swine farms.
The efficacy of current PRRS modified live vaccines could be enhanced with the addition of adjuvants. In fact, a recent study demonstrated that the addition of adjuvant to MLV led to broadened cross-protection to PRRSV field isolates and reduced lung and lymph organ damage [7], suggesting that adjuvant addition to MLV would be an effective way to reduce PRRS disease. However, Mycobacterium tuberculosis whole cell lysate was used as the adjuvant in that study and it would be too expensive for food animal vaccine markets. On the other hand, more cost-effective commercially available Gel01 adjuvant has been proven to be easy to use and stable in a variety of veterinary vaccines. The addition of Gel01 adjuvant to PRRS MLV has been reported previously [9]. In this report, Gel01 adjuvant could improve the efficacy of PRRS MLV even with half of the antigen load. Gel01- adjuvanted PRRS MLV also reduced viremia after challenge and generated equivalent ELISA antibody titers as MLV alone. Better protection was shown by reduced duration of hyperthermia and lung pathology score with administration of Gel01-adjuvanted PRRS MLV after viral challenge.
Our study shows similar results in that Gel01-MLV was able to better protect pigs challenged with VR-2332 than pigs vaccinated with MLV alone. However, when pigs were challenged with a heterologous strain of PRRSV (MN184A), addition of Gel01 adjuvants did not enhance protection (Figure 1). The amino acid similarity of structural proteins between MLV and VR-2332 is more than 99.2%, yet MN184A share only 89.4%, which may have contributed to lack of cross-protection after MN184A challenge. Therefore, Gel01 may not be an ideal adjuvant for all strains of PRRSV, but rather is strain-specific in the ability to enhance the protective properties of MLV.
In our study, pigs vaccinated with Gel01-MLV developed higher titers of PRRSV-specific antibodies after vaccination and VR-2332 challenge, as measured by IDEXX ELISA (Figure 2). However, these ELISA antibodies are non-neutralizing antibodies that are mainly directed towards the nucleocapsid (N) protein, and did not provide animals with any protection against PRRSV infection [14]. In contrast, PRRSV structural proteins are reported to induce protective neutralizing antibody (NA) and PRRSV-specific cellular immune response after PPRSV infection [15]. To further evaluate the immunological mechanisms of Gel01-mediated adjuvanticity, we found that pigs vaccinated with Gel01-adjuvanted MLV, as opposed to MLV alone, generated higher NA titer when challenged with VR-2332 (Figure 2). Interestingly, pigs vaccinated with Gel01-MLV showed higher NA titer to both VR-2332 and MN184A than that in pigs vaccinated with MLV alone after they were challenged with PRRSV MN184A. Similar results have also been observed on other recently isolated field strains of PRRSV (unpublished data). These results are consistent with the speculation that the prime (vaccination-MLV) and boost with heterologous PRRSV strain (challenge-MN184A) are able to generate higher NA titers, a concept which has already been shown to occur in influenza virus vaccination [16].
There are several immunomodulatory cytokines that are believed to be responsible for the clearance of PRRS virus. Specifically, vaccine-mediated upregulation of pronflammatory cytokine IFN-γ has been suggested to be important in the combat against PRRSV infection [17]. Interestingly, in our study, pigs receiving Gel01 adjuvanted MLV were better protected against homologous PRRSV infected than MLV vaccinated pigs; however, Gel01-MLV pigs had decreased IFN-γ (Figure 3). These results suggest that IFN-γ may be playing a negative role in protecting pigs from disease and agents that can reduce IFN-γ levels in vaccinated pigs and may lead to better protection. Additionally, pigs vaccinated with the MLV alone had increased IL-10 production as compared to unvaccinated animals and the addition of Gel acted to decrease IL-10 to levels to at or below unvaccinated anils (Figure 3). During PRRSV infection, a significant corlation has previously been observed between the inability to effectively protect against disease and the increased expression of cytokine IL10. This could be in part due to IL10-mediated reduction of IFN-α, IFN-γ, IL-12 and TNF-α expression, cytokines involved in dampening the cellular immune response [5]. Therefore, our results suggest that Gel01 adjuvant may act to enhance the protective properties of MLV by decreasing IL-10 production.
The expression of IL-10 is mainly regulated by T-relatory cells, which consist of a small subpopulation of T lymphocytes [18]. Consistent with IL-10 expression, we found that the frequency of T regulatory cells in Gel01- juvanted vaccinated pigs was dramatically reduced in the TBLNs and lung MNCs (Figure 4). Therefore the reduced T-regulatory cell population could have contributed to the decreased expression of IL-10 in pigs after vaccination with Gel01-MLV and challenge with PRRSV.
In summary, our results show that addition of Gel01 adjuvant to PRRSV modified live vaccine can confer increased protection to homologous but not heterologous PRRSV challenge, presumably through higher titers of ELISA and neutralizing antibodies and reduced IFN-γ and IL-10 cytokine production. Therefore, the commercially available Gel01 adjuvant may be a useful tool in improving the efficacy of live PRRSV vaccines.
5. Acknowledgements
We thank Dr. Brooke Bloomberg and the rest of the Comparative Medicine staff at Kansas State University for their technical help. We would also like to thank Nanhua Chen, Theresa Quintana, and Sarah Ebarb for their help in handling our animals. This research was supported in part by KBA-CBRI 611310, NIH R21 AI085416, and a research grant from Kansas State University Research Foundation.
REFERENCES
- R. J. Chand, B. R. Trible and R. R. Rowland, “Pathogenesis of Porcine Reproductive and Respiratory Syndrome Virus,” Current Opinion Virology, Vol. 2, No. 3, pp. 256- 263. doi:10.1016/j.coviro.2012.02.002
- United States Department of Agriculture, “PRRS Seroprevalence on U.S. Swine Operations,” 2009. http://www.aphis.usda.gov/animal_health/nahms/swine/downloads/swine2006/Swine2006_is_PRRS.pdf
- T. G. Kimman, L. A. Cornelissen, R. J. Moormann, J. Rebel and N. S. Zurmieden, “Challenges for Porcine Reproductive and Respiratory Syndrome Virus (PRRSV) Vaccinology,” Vaccine, Vol. 27, No. 28, 2009, pp. 3704- 3718.
- W. Charerntantanakul, R. Platt, W. Johnson, M. Roof, E. Vaughn and J. A. Roth, “Immune Responses and Protection by Vaccine and Various Vaccine Adjuvant Candidates to Virulent PRRSV,” Veterinary Immunology and Immunopathology, Vol. 109, No. 1-2, 2006, pp. 99-115. doi:10.1016/j.vetimm.2005.07.026
- R. Thanawongnuwech and S. Suradhat, “Taming PRRSV: Revisiting the Control Strategies and Vaccine Design,” Virus Research, Vol. 154, No. 1-2, 2010, pp. 133-140. doi:10.1016/j.virusres.2010.09.003
- Y. W. Huang and X. J. Meng, “Novel Strategies and Approaches to Develop the Next Generation of Vaccines against Porcine Reproductive and Respiratory Syndrome Virus (PRRSV),” Virus Research, Vol. 154, No. 1-2, 2010, pp. 141-149. doi:10.1016/j.virusres.2010.07.020
- B. Binjawadagi, V. Dwivedi, C. Manickam, J. B. Torrelles and G. J. Renukaradhya, “Intranasal Delivery of an Adjuvanted Modified Live Porcine Reproductive and Respiratory Syndrome Virus Vaccine Reduces ROS Production,” Viral Immunology, Vol. 24, No. 6, 2011, pp. 475- 482. doi:10.1089/vim.2011.0040
- S. I. Jang, H. S. Lillehoj, S. H. Lee, K. W. Lee, M. S. Park, G. R. Bauchan, E. P. Lillehoj, F. Bertrand, L. Dupuis and S. Deville, “Immunoenhancing Effects of Montanide ISA Oil-Based Adjuvants on Recombinant Coccidia Antigen Vaccination against Eimeria Acervulina Infection,” Veterinary Parasitology, Vol. 172, No. 3-4, 2010, pp. 221-4218. doi:10.1016/j.vetpar.2010.04.042
- S. Dveille, J. Arous, G. Ionkoff, F. Kukushkin, T. Baybikov, V. Borisov and L. Dupius, “Load Reduction in Live PRRS Vaccines Using Oil and Polymer Adjuvants,” Procedia in Vaccinology, 2012, pp. 134-140. doi:10.1016/j.provac.2012.04.018
- J. Beyer, D. Fichtner, H. Schirrmeier, U. Polster, E. Weiland and H. Wege, “Porcine Reproductive and Respiratory Syndrome Virus (PRRSV): Kinetics of Infection in Lymphatic Organs and Lung,” Journal of Veterinary Medcine, Series B, Vol. 47, No. 1, 2000, pp. 9-25.
- S. Dotti, R Villa, E. Sossi, G. Guadagini, F. Salvini, M. Ferrari and M. Amadori, “Comparative Evaluation of PRRS Virus Infection in Vaccinated and Naïve Pigs,” Research in Veterinary Science, Vol. 90, No. 2, 2011, pp. 218-225. doi:10.1016/j.rvsc.2010.06.011
- W. Charerntantanakul, R. Platt and J. A. Roth, “Effects of Porcine Reproductive and Respiratory Syndrome VirusInfected Antigen-Presenting Cells on T Cell Activation and Antiviral Cytokine Production,” Viral immunology, Vol. 19, No. 4, 2006, pp. 646-661. doi:10.1089/vim.2006.19.646
- B. Pulendran and R. Ahmed, “Immunological Mechanisms of Vaccination,” Nature Immunology, Vol. 6, No. 12, 2011, pp. 509-517.
- E. Mateu and I. Diaz, “The Challenge of PRRS Immunology,” Veterinary Journal, Vol. 177, No. 3, 2008, pp. 345-351. doi:10.1016/j.tvjl.2007.05.022
- G. J. Renukaradhya, V. Dwivedi , C. Manickam , B. Binjawadagi and D. Benfield , “Mucosal Vaccines to Prevent Porcine Reproductive and Respiratory Syndrome: A New Perspective,” Animal Health Research Review, Vol. 13, No. 1, 2012, pp. 21-37 doi:10.1017/S1466252312000023
- C. J. Wei, J. C. boyington, P. M. Mctamney, W. P. Kong, M. B. Pearce, L. Xu, H. Andesen, S. Rao, T. M. Tumpey, Z. Y. Yang and G. J. Nabel, “Induction of Broadly Neutralizing H1N1 Influenza Antibodies by Vaccination,” Science, Vol. 329, No. 5995, 2010, pp. 1060-1064. doi:10.1126/science.1192517
- Z. Xiao, L. Batista, S. Dee, P. Halbur and M. P. Murtaugh, “The Level of Virus-Specific T-Cell and Macrophage Recruitment in Porcine Reproductive and Respiratory Syndrome Virus Infection in Pigs Is Independent of Virus Load,” Journal of Virology, Vol. 78, No. 11, 2004, pp. 5923-5933
- S. suradhat and R. Thanawongnuwech, “Upregulation of Interleukin-10 Gene Expression in the Leukocytes of Pigs Infected with Porcine Reproductive and Respiratory Syndrome Virus,” Journal of General Virology, Vol. 84, No. 10, 2003, pp. 2755-2760. doi: 10.1099/vir.0.19230-0
NOTES
*Corresponding author.

