Agricultural Sciences
Vol.07 No.01(2016), Article ID:63111,10 pages
10.4236/as.2016.71001
Silicon Mediated Arsenic Reduction in Rice by Limiting Its Uptake
Muhammad Mohsin Raza1, Sami Ullah2, Zulfiqar Ahmad3, Sohaib Saqib3*, Sarfraz Ahmad2, Hafiz Muhammad Bilal3, Farman Wali3
1Arid Zone Research Institute (PARC) Bahawalpur, Pakistan
2Department of Agronomy, University of Agriculture, Faisalabad, Pakistan
3Institute of Soil and Environmental Sciences, University of Agriculture, Faisalabad, Pakistan

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 26 November 2015; accepted 25 January 2016; published 28 January 2016
ABSTRACT
This study was planned to examine the effects of exogenous silicon supply on growth parameters and arsenic accumulation level in rice. The experiment was conducted in the wire house of Saline Agriculture Research Centre, Institute of Soil and Environmental Science, University of Agriculture Faisalabad. The study was comprised of treatments viz: control; (100 µM Arsenic); (200 µM Arsenic); (5 mM Silicon); (5 mM Silicon + 100 µM Arsenic) and (5 mM Silicon + 200 µM Arsenic). Results revealed that maximum shoot fresh weight, shoot dry weight, root fresh weight and root dry weight were observed in (5 mM Si) solution. In the same way, maximum number of tillers was also recorded in (5 mM Si) solution; while silicon application failed to alleviate arsenic concentration of rice genotype.
Keywords:
Silicon, Arsenic, Rice, Growth, As Concentration in Plant

1. Introduction
From recent past, heavy metal contamination is considered as a serious soil problem that negatively affects plant growth and productivity worldwide. Arsenic is one of the highly toxic heavy metals and [1] [2] it is considered as the most significant pollutant due to its large solubility in water [3] , however, it does not dissolve in bases [4] . Arsenic is a widespread environmental contaminant that is becoming the cause of serious health problems in humans and animals. Soils and water bodies which are contaminated with Arsenic are major source of arsenic entry in food chain. Groundwater of South East Asia such as China, India and Bangladesh is contaminated [5] . In Bangladesh, India, China, Mexico, Chile, Argentina, Nepal, Srilanka and Pakistan, As contamination of drinking water supplies is a major public health emergency [6] . Arsenic concentrations in drinking water in these countries have been found to exceed 1000 µg∙L−1 (mainly from contaminated well water) [7] . Chronic and acute contact to As results different kind of diseases, which mainly include respiratory diseases, non-pitting edema, gastro-intestinal diseases, liver and cardiovascular disorder, and in the long run cancer and death [8] .
Silicon is considered beneficial and valuable for plant growth, especially under several biotic stresses (e.g. plant diseases and pests) and a biotic (e.g. drought, salt and metal toxicity) stress [9] . Silicon also decreases concentration of several metals in rice including Zn, Cd, As and Al [10] . Silicon has been considered important in toxicity reduction in plants through absorbing heavy metals in roots and minimizing their transportation to shoots [10] . Silicon deposits the SiO2 in the apoplast of the leaf surface and on the roots, which forms barrier to the flow of metallic ions [11] . Silicon decreases As translocation to rice grains and straw [12] . Silicon increased the antioxidant enzyme activity and lowered the As levels in rice plants when it was added in growth medium [13] . One of the major effects of silicon on the reduction of metal toxicity is the reduction of metal uptake and transport in plants. It is reported by many researchers that silicon application increased tolerance to toxic metals in many plants due to reduction in uptake and translocation of metals. Soil application of silicon decreased arsenic concentration in straw, husk, flag leaf and grains of rice plants [14] .
Keeping these backgrounds in consideration, experiment was planned with following objectives:
Ø To monitor the effect of As on growth of rice crop;
Ø To assess the effect of Si on As accumulation and concentration in rice crop.
2. Material and Methods
2.1. Experimental Location
The experiment was conducted in the wire house of Saline Agriculture Research Centre, Institute of Soil and Environmental Science, University of Agriculture Faisalabad. The wire house has a glass covered roof, sides with iron wire screen and no control of humidity, temperature and light.
2.2. Experimental Design
Completely Randomized Design (CRD) factorial was used during course of study.
2.3. Seed Source
The seed of rice genotype KS-133 was taken from Saline Agriculture Research Centre, Institute of Soil and Environmental Sciences University of Agriculture Faisalabad.
2.4. Arsenic and Silicon Source
Arsenic toxicity was artificially produced by using sodium arsenate and sodium arsenite, while silicon source was silicic acid.
2.5. Nursery Raising and Transplantation
Seed of rice variety was germinated in sand filled iron tray. Optimum moisture was maintained. After germination the uniform size seedlings were transplanted in foam-plugged holes of polystyrene sheets floating over ½ strength Hoagland’s nutrient solution [15] . Hundred liter tubs were used in the experiment.
2.6. Growth Conditions and Salt Imposition
The pH of the solution was monitored by using pH meter and adjusted at 6 ± 0.5, with the required addition of acid (HCl) or base (NaOH). Aeration was provided for eight hours daily by using aeration pump. Solutions were changed weekly. Each cultivar was replicated four times.
2.7. Treatment Detail
There were eight treatments including control having only ½ strength Hoagland solution.
T1: Control;
T2: (100 µM Arsenic);
T3: (200 µM Arsenic);
T4: (5 mM Silicon);
T5: (5 mM Silicon + 100 µM Arsenic);
T6: (5 mM Silicon + 200 µM Arsenic).
2.8. Harvesting and Data Collection
Plants were harvested after six weeks of transplanting. Plants were washed with distilled water and dried with blotting paper. Data were recorded for growth and ionic parameters. Plant leaves were analyzed for arsenic concentration.
2.9. Growth Parameters
Length and weight was measured using scale and electric balance respectively.
Following growth parameters were recorded:
1- Root fresh weight;
2- Shoot fresh weight;
3- Shoot dry weight;
4- Root dry weight;
5- Number of tillers.
2.10. Ionic Analysis
Arsenic Concentrations in Plant Material
Arsenic concentration in plant samples was determined after wet-oxidation using spectrophotometer following the method of [16] . According to the normalized protocol, 1ml ascorbic acid solution and 2 ml reagent A are successively added to a 40 ml sample aliquot in a 50 ml volumetric flask, and the volume completed by de-io- nized water. A blank is prepared according to the same procedure using the appropriate volume of de-ionized water. The analysis is carried out in 1 cm quartz cells with an Agilent 8453 UV visible spectrometer. Complex formation was carried out at constant controlled temperature (20˚C ± 1˚C). The optimal complex formation time was determined for an As (V) concentration of 2 × 10−5 M. At regular time intervals, solutions were analyzed for complex formation through absorbance. The most sensitive detection wavelength was determined at the same time. The action of various parameters upon complex formation was tested. The complex was allowed to form in daylight or in darkness and at various temperatures between 5˚C and 50˚C. Silicate and sulphate influence upon complex formation were tested with concentrations of 10 and 35 mg∙L−1 for silicate and sulphate, respectively. The detection limit of this method under the best conditions was then studied with a 10 cm glass cell. As (V) concentration was progressively decreased and repeatability was checked until an unstable or undetectable signal was obtained.
2.11. Statistical Analysis
All the presented data in the study are the mean of six replicates and standard errors. Analysis of variance was performed in CRD using factorial design [17] with a statistical package Statistic version 8.1. Significant differences among treatments were considered at the P ≤ 0.05 levels. Comparison among the treatment will be will be analyzed using LSD test.
3. Results and Discussions
3.1. Effect of Si and As application on Shoot Fresh Weight (g)
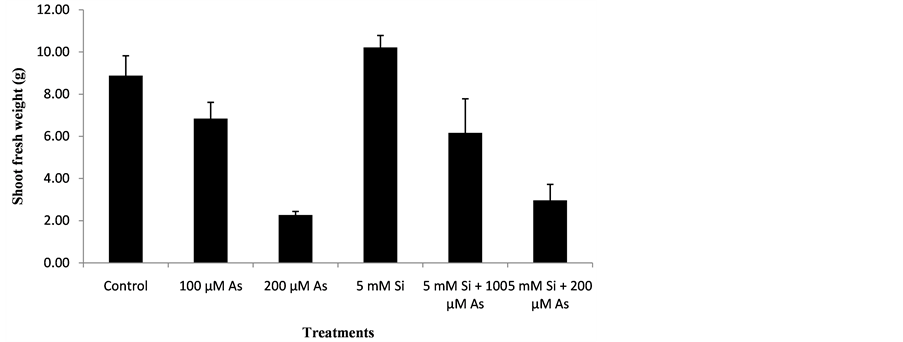
The data regarding the effect of Si and As application on shoot fresh weight of plants was analyzed statistically at P ≤ 0.05. The statistical interpretation showed significant effect of treatments on shoot fresh weight of rice genotype. Data regarding the effect of Si and As on shoot fresh weight of plants is mentioned below in the Figure 1.
Figure 1. Effect of Si and As application on shoot fresh weight (g).
At harvest time, maximum shoot fresh weight was recorded in treatment T4 followed by T1, T2, T5, T6 and T3. Treatment T4 (5 mM Si) showed Maximum shoot fresh weight because in that treatment silicon application was done and no arsenic toxicity was present. Due to silicon application maximum shoot fresh weight was observed in treatment 4 (5 mM Si). In treatment T4, there was 47% increase in shoot fresh weight as compared to control. Under arsenic stress condition, treatment T2 (100 µM As) showed higher shoot fresh weight as compared to treatment T3 (200 µM As) because less concentration (100 µM) of arsenic was present in treatment T2. In treatment T2, there was 23% reduction in shoot fresh weight as compared to control. When silicon and arsenic both were applied together in treatment T5 and T6, treatment T5 (5 mM Si + 100 µM As) showed higher shoot fresh weight as compared to treatment T6 (5 mM Si + 200 µM As) because in that treatment arsenic toxicity of (100 µM) was reduced by silicon application. In treatment T5, 30% reduction in shoot fresh weight was observed as compared to control. In treatment T6 (5 mM Si + 200 µM As) arsenic concentration was less reduced by silicon application. In treatment T6, 66% reduction in shoot fresh weight was recorded as compared to control. Minimum shoot fresh weight was recorded in treatment T3 (200 µM As) because in that treatment higher arsenic concentration of (200 µM) was present. In treatment T3, 74% reduction in shoot fresh weight was recorded as compared to control. Silicon is very effective in alleviating heavy metal stresses. Due to silicon application plant growth increases so shoot fresh weight also increases [18] . The results were similar to that of other scientists describe as below. [19] reported that when 3mM silicate was added exogenously to nutrient solution, it results in increase in growth of shoots. They also concluded that additional application of silicon in nutrient solution increases the shoot fresh weight. [20] concluded that increasing concentrations of As at early growth stages of pea plants caused a gradual decrease in shoot fresh weight. Si application was very effective in alleviating the toxic effects of arsenic on plant growth such as shoot fresh weight.
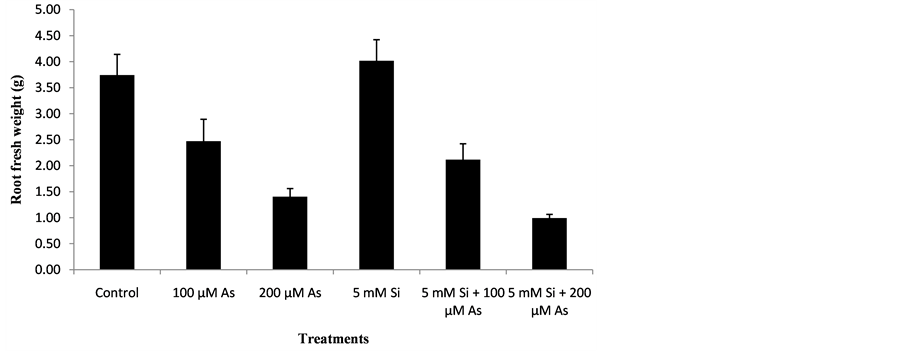
3.2. Effect of Si and As Application on Root Fresh Weight (g)
The effect of treatments for root fresh weight was significant and the performance of all the genotype was statistically significant (P < 0.05). The data regarding root fresh weight of rice is shown by the Figure 2. Graph showed the significant reduction in root fresh weight of rice genotype with increasing arsenic stress as compared to control. Similar trend was shown in case of treatments T4, T5, T6. The maximum root fresh weight was observed in treatment T4 (5 mM Si) in which 5 mM Silicon was added and no arsenic stress was present. In treatment T4, 34% increase in root fresh weight was observed as compared to control. The minimum root fresh weight was recorded from treatment T6 (5 mM Si + 200 µM As) in which both silicon and arsenic were present. In treatment T6, Silicon at a concentration of 5mM had less effective in reducing arsenic toxicity of 200 µM. In Treatment T6, 82% reduction in root fresh weight was recorded as compared to control. Treatment T5 (5mM Si + 100 µM As) showed higher root fresh weight compared with treatment T6 because in that treatment less concentration of arsenic (100 µM) was present so silicon concentration of (5mM) resulted in higher root fresh weight. In Treatment T5, 25% reduction in root fresh weight was recorded as compared to control .In the presence of arsenic toxicity, Treatment T2 (100 µM As) showed higher root fresh weight as compared to Treatment
Figure 2. Effect of Si and As application on root fresh weight (g).
T3 (200 µM As) because in treatment T2 Less arsenic toxicity of (100 µM) was present. In Treatment T2, 33% reduction in root fresh weight was recorded as compared to control. Heavy metal toxicity reduces the plant growth there by reducing root fresh weight. Silicon causes increase in plant biomass. Silicon improves the growth condition of plants. Silicon reduces the heavy metal uptake by plants so root fresh weight of crop plant increases [21] . The results were similar to that of other scientists describe as below. [22] reported that arsenic toxicity reduced the root fresh weight. Application of silicon resulted in higher root fresh weight. [23] reported that high concentration of arsenic reduced the root fresh weight. Addition of silicon to nutrient solution resulted in higher root fresh weight.
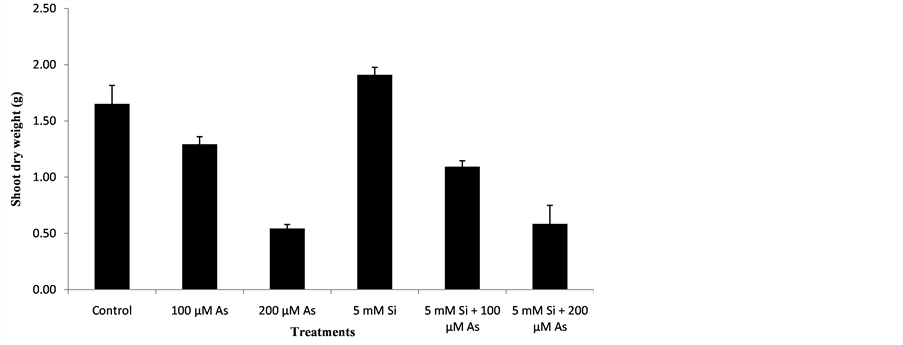
3.3. Effect of Si and As Application Shoot Dry weight (g)
The effect of treatments for shoot dry weight was significant and the performance of all the genotype was statistically significant (P < 0.05). The data regarding shoot dry weight of rice is shown by the Figure 3. Graph showed the significant reduction in shoot dry weight of rice genotype with increasing arsenic stress as compared to control. Similar trend was shown in case of treatments T4, T5, T6. The maximum shoot dry weight was observed in treatment T4 (5 mM Si) in which 5mM Silicon was added and no arsenic stress was present. In treatment T4, 56% increase in shoot dry weight was observed as compared to control. The minimum shoot dry weight was recorded from treatment T3 (200 µM As) in which arsenic concentration of 200 µM was present. In Treatment T3, 66% reduction in shoot dry weight was recorded as compared to control In treatment T6 (5 mM Si + 200 µM As), Silicon at a concentration of 5mM had less effective in reducing arsenic toxicity of 200 µM. In Treatment T6, 64% reduction in shoot dry weight was recorded as compared to control. Treatment T5 (5 mM Si + 100 µM As) showed more shoot dry weight compared with treatment T6 because in that treatment less concentration of arsenic (100 µM) was present so silicon concentration of (5 mM) resulted in higher shoot dry weight. In Treatment T5, 13% reduction in shoot dry weight was recorded as compared to control .In the presence of arsenic toxicity, Treatment T2 (100 µM As) showed more shoot dry weight as compared to Treatment T3 (200 µM As) because in treatment T2 Less arsenic toxicity of (100 µM) was present. In Treatment T2, 13% reduction in shoot dry weight was recorded as compared to control. Heavy metal toxicity reduces the plant growth there by reducing shoot dry weight. The results were similar to that of other scientists describe as below. [24] reported that high concentration of arsenic decreased the shoot dry weight. Shoot dry weight was increased by silicon application. [25] reported that when maize seedlings were exposed to different As levels, shoot dry weight was reduced due to arsenic toxicity. Silicon improved the growth of crop thereby increasing shoot dry weight. [26] reported that rice seedlings that were treated with silica gel exhibited high shoot dry weight.
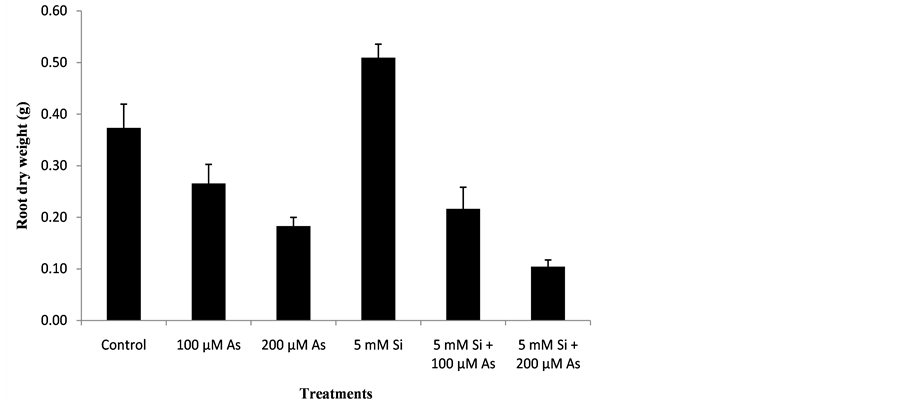
3.4. Effect of Si and As Application on Root Dry Weight (g)
The data regarding the effect of Si and As application on root dry weight of plants was analyzed statistically at P ≤ 0.05. The statistical interpretation showed significant effect of treatments on root dry weight of rice genotype. Data regarding the effect of Si and As on root dry weight of plants is mentioned below in the Figure 4.
Figure 3. Effect of Si and As application shoot dry weight (g).
Figure 4. Effect of Si and As application on root dry weight (g).
At harvest time, maximum root dry weight was recorded in treatment T4 followed by T1, T2, T5, T6 and T3. Treatment T4 (5 mM Si) showed Maximum root dry weight because in that treatment silicon application was done and no arsenic toxicity was present. Due to silicon application maximum root dry weight was observed in treatment 4 (5 mM Si). In treatment T4, there was 79% increase in root dry weight as compared to control. Under arsenic stress condition, treatment T2 (100 µM As) showed higher root dry weight as compared to treatment T3 (200 µM As) because less concentration (100 µM) of arsenic was present in treatment T2. In treatment T2, there was 28% reduction in root dry weight as compared to control. When silicon and arsenic both were applied together in treatment T5 and T6, treatment T5 (5 mM Si + 100 µM As) showed higher root dry weight as compared to treatment T6 (5 mM Si + 200 µM As) because in that treatment arsenic toxicity of (100 µM) was reduced by silicon application. In treatment T5, 38% reduction in root dry weight was observed as compared to control. In treatment T6 (5 mM Si + 200 µM As) arsenic concentration was less reduced by silicon application. Minimum root dry weight was recorded in treatment T6 (5 mM Si + 200 µM As) because in that treatment higher arsenic concentration of (200 µM) was present. In treatment T6, 72% reduction in root dry weight was recorded as compared to control. In treatment T3 (200 µM As), 50% reduction in root dry weight was recorded as compared to control. The results were similar to that of other scientists describe as below. [27] reported that arsenic toxicity reduced the root dry weight of maize seedlings. Root dry weight was increased after silicon application. [28] reported that reduction in root dry weight of oilseed was noticed when exposed to arsenic stress. Silicon application increased the root dry weight.
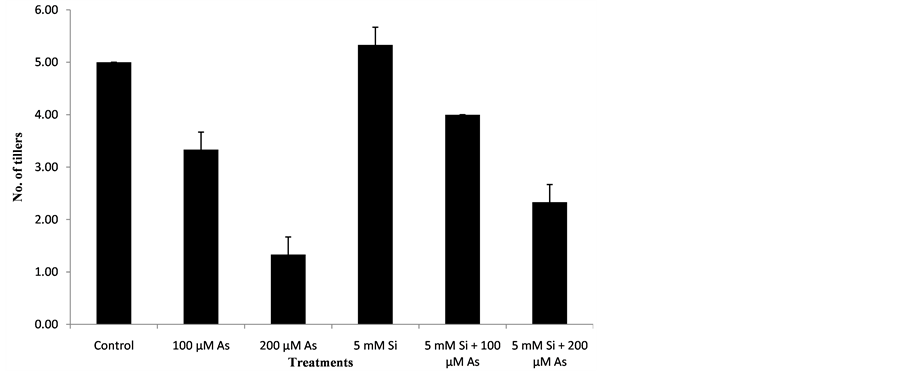
3.5. Effect of Si and As Application on Number of Tillers per Plant
The data regarding the effect of Si and As application on number of tillers per plants was analyzed statistically at P ≤ 0.05. The statistical interpretation showed significant effect of treatments on numbers of tillers of rice genotype. Data regarding the effect of Si and As on numbers of tillers of plants is mentioned below in the Figure 5.
At harvest time, maximum number of tillers per plant were recorded in treatment T4 followed by T1, T5, T2, T6 and T3. Treatment T4 (5 mM Si) showed Maximum number of tillers per plant because in that treatment silicon application was done and no arsenic toxicity was present. Due to silicon application number of tillers was increased in treatment T4. In treatment T4, there was 26% increase in number of tillers as compared to control. Under arsenic stress condition, treatment T2 (100 µM As) showed more number of tillers as compared to treatment T3 (100 µM As) because less concentration (100 µM) of arsenic was present in treatment T2. In treatment T2, there was 33% decrease in number of tillers as compared to control. When silicon and arsenic both were applied together in treatment T5 and T6, treatment T5 (5 mM Si + 100 µM As) showed more number of tillers as compared to treatment T6 (5 mM Si + 200 µM As) because in that treatment arsenic toxicity of (100 µM) was reduced by silicon application. In treatment T5, there was 20% decrease in number of tillers as compared to control. In treatment T6 arsenic concentration of (200 µM) was less reduced by silicon application therefore it showed less number of tillers as compared to treatment T5. In treatment T6, there was 53% decrease in number of tillers as compared to control. Minimum number of tillers were recorded in treatment T3 (200 µM As) because in that treatment higher arsenic concentration of (200 µM) was present. In treatment T3, there was 73% decrease in number of tillers as compared to control. Due to presence of arsenic in excess amount, plant growth decreases due to which the number of tillers of a plant was reduced. Silicon increases growth parameters of crop. Application silicon also increases number of tillers per plant due to which yield of crop is increased [16] . The results were similar to that of other scientists describe as below. [29] reported that addition of silicon considerably increased the number of boll of cotton crop. Silicon application increased number of tiller. Tiller production was reduced arsenic toxicity.
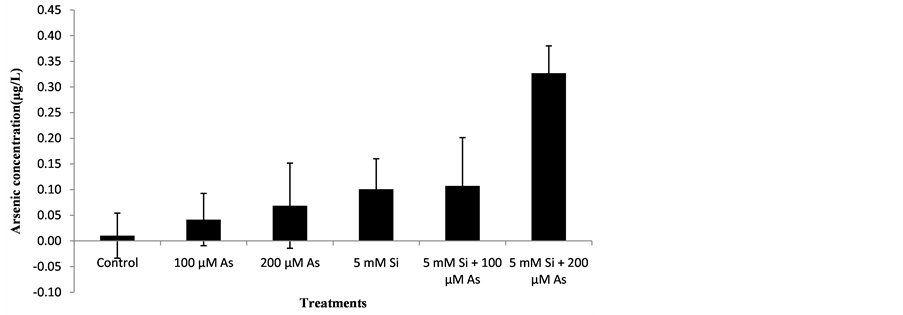
3.6. Effect of Si and As Application on Arsenic Concentration in Plant
The data regarding the effect of Si and As application on arsenic concentration of plants was analyzed statistically at P ≤ 0.05. The statistical interpretation showed non-significant effect of treatments on arsenic concentration of rice genotype. Data regarding the effect of Si and As on arsenic concentration of plants is mentioned below in the Figure 6.
At harvest time, maximum arsenic concentration was recorded in treatment T6 followed by T5, T4, T3, T2 and T1. Treatment T6 (5 mM Si + 200 µM As) showed maximum arsenic concentration because in that treatment silicon application was done and arsenic toxicity was also present. Minimum arsenic concentration was recorded in treatment T1 (control). Arsenic concentration was minimum in control plants. Arsenic concentration in shoot differs from plant, genotype to genotype and even species to species.
Figure 5. Effect of Si and As application on number of tillers per plant.
Figure 6. Effect of Si and As application on Arsenic concentration in plant.
The results of the present study were in contrast with others as reported by [30] who found As concentrations in rice seedlings differed among 20 genotypes. These differences may be related to the ability of As(V) uptake, but not related to As(III) efflux since root As concentrations of wheat seedlings were positively correlated (not significant) to As(V) uptake, and significantly (P < 0.05) negatively correlated with relative As(III) efflux. Generally, As(V) is taken up via the P transporters in high plants [31] . In plant roots, As(V) is rapidly reduced to As(III) by the arsenate reductase [32] , then a large proportion of the As(III) was extruded to medium [33] .
4. Conclusions
Parameters like fresh and dry shoot weight, fresh and dry root weight, number of tillers and As concentration in rice plant were studied. Each parameter was evaluated statistically and results were illustrated as below:
・ Silicon application increased shoot fresh weight and shoot dry weight;
・ Silicon application also effected root fresh weight and root dry weight;
・ The number of tillers per plant was also affected under the Si application;
・ As concentration in plant was not affected by Si supply.
Cite this paper
MuhammadMohsin Raza,SamiUllah,ZulfiqarAhmad,SohaibSaqib,SarfrazAhmad,Hafiz MuhammadBilal,FarmanWali, (2016) Silicon Mediated Arsenic Reduction in Rice by Limiting Its Uptake. Agricultural Sciences,07,1-10. doi: 10.4236/as.2016.71001
References
- 1. Toppi, D., Singh, L. and Gabrielli, R. (1999) Responses to Cadmium in Higher Plants. Environmental Experimental Botany, 41, 105-130.
http://dx.doi.org/10.1016/S0098-8472(98)00058-6 - 2. AlKhedhairy, A.A., Al-Rokayan, S.A. and Al-Misned, F.A. (2001) Cadmium Toxicity and Cell Stress Response. Pakistan Journal of Biological Science, 4, 1046-1049.
http://dx.doi.org/10.3923/pjbs.2001.1046.1049 - 3. Pinto, A.P., Mota, A.M., devarennes, A. and Pinto, F.C. (2004) Influence of Organic Matter on the Uptake of Cadmium, Zinc, Copper and Iron by Sorghum Plants. Science of the Total Environment, 326, 239-247.
http://dx.doi.org/10.1016/j.scitotenv.2004.01.004 - 4. Borsari, M. (2011) Cadmium: Inorganic and Coordination Chemistry. In: Encyclopaedia of Inorganic and Bioinorganic Chemistry, John Wiley and Sons Ltd., New York.
http://dx.doi.org/10.1002/9781119951438.eibc0029 - 5. Chowdhury, T.R., Basu, G.K., Mandal, B.K., Samanta, G., Chowdhury, U.K., Chanda, C.R., Lodh, D., Lal, R.S., Saha, K.C. and Roy, S. (1999) Arsenic Poisoning in Ganges Delta. Nature, 401, 545-546.
- 6. Petrusevski, B., Sharma, S., Schippers, J.C. and Shordt, K. (2007) Arsenic in Drinking Water. Journal of International Water Sanitation Kent, 17, 36-44.
- 7. Samadder, S.R. (2010) Impact of Arsenic Pollution in Drinking Water on Life Expectancy: A GIS Study. Journal of Civil Engineering, 14, 681-691.
http://dx.doi.org/10.1007/s12205-010-0892-z - 8. Kapaj, S., Peterson, H., Liber K. and Bhattacharya, P. (2006) Human Health Effects from Chronic Arsenic Poisoning a Review. Journal of Environmental Sciences and Health, 41, 2399-2428.
http://dx.doi.org/10.1080/10934520600873571 - 9. Liang, Y., Wong, J.W.C. and Wei, L. (2004) Silicon Mediated Enhancement of Arsenic Tolerance in Maize (Zea mays L.) Grown in Arsenic Contaminated Soil. Chemotherapy, 58, 475-483.
- 10. Shi, X., Chaochun, Z., He, W. and Fusuo, Z. (2005) Effect of Si on the Distribution of Cd in rice Seedlings. Journal of Plant and Soil, 272, 53-60.
http://dx.doi.org/10.1007/s11104-004-3920-2 - 11. Lux, A., Luxova, M., Hattori, T., Inanaga, S. and Sugimoto, Y. (2002) Silicification in Sorghum (Sorghum bicolor) Cultivars with Different Drought Tolerance. Journal of Physiology of Plant, 115, 87-92.
http://dx.doi.org/10.1034/j.1399-3054.2002.1150110.x - 12. Ali, W., Stanisla, V., Isayenkov, V.S., Zhao, F. and Maathuis, F.L.M. (2009) Arsenite Transport in Plants. Journal of Cellular Molecular Life Sciences, 66, 29-39.
http://dx.doi.org/10.1007/s00018-009-0021-7 - 13. Tripathi, P., Tripathi, R.D., Singh, R.P., Dwivedi, S., Goutam, D., Shri, M., Prabodh, K.T. and Chakrabarty, D. (2013) Silicon Mediates Arsenic Tolerance in Rice (Oryza sativa L.) through Lowering of Arsenic Uptake and Improved Antioxidant Defence System. Journal Ecological. Engineering, 52, 96-103.
- 14. Fleck, A.T., Mattusch, J. and Schenk, M.K. (2013) Silicon Decreases the Arsenic Level in Rice Grains by Limiting the Arsenite Transport. Journal Plant Nutrition Soil Science, 176, 785-794.
http://dx.doi.org/10.1002/jpln.201200440 - 15. Hoagland, D.R. and Arnon, D.I. (1950) The Water Culture Method for Growing Plant Without Soil. California Agriculture Experimental Station, Circular, 347, 39.
- 16. Lenoble, V., Deluchat, V., Serpaud, B. and Bollinger, J. (2003) Arsenite Oxidation and Arsenate Determination by the Molybdene Blue Method. Talanta Journal, 61, 267-276.
http://dx.doi.org/10.1002/jpln.201200440 - 17. Steel, R.G.D., Torrie, J.H. and Dicky, D.A. (1998) Principles and Procedures of Statistics. 2nd Edition, McGraw Hill Book Co., USA.
- 18. Liang, Y.C., Shen, Q.R. and Ma, T.S. (2003) Effects of Silicon on Salinity Tolerance of Two Barley Cultivars. Journal Plant Nutrition, 19, 173-183.
http://dx.doi.org/10.1080/01904169609365115 - 19. Gong, H.J., Randall, D.P. and Flowers, T.J. (2006) Silicon Deposition in the Root Reduces Sodium Uptake in Rice Seedlings by Reducing Bypass Flow. Journal of Plant Cell Environment, 111, 1-9.
http://dx.doi.org/10.1111/j.1365-3040.2006.01572.x - 20. Popova, L., Maslenkova1, L., Yordanova1, R., Krantev, A., Szalai, G. and Janda, T. (2008) Salicylic Acid Protects Photosynthesis against Arsenic Toxicity in Pea Plants. Journal General Applied Plant Physiology, 34, 133-148.
- 21. Ma, J.F., Tamal, K., Yamaji, N., Mitani, N., Konishi, S., Katuhara, M., Ishiguru, M. and Yano, M. (2006) A Silicon Transporter in Rice. Nature, 440, 688-691.
http://dx.doi.org/10.1038/nature04590 - 22. Ammar, W.B., Nouairi, I., Zarrouk, M. and Jemal, F. (2007) Cadmium Stress Induces Changes in the Lipid Composition and Biosynthesis in Tomato (Lycopersicon esculentum Mill.) Leaves. Plant Growth Regulation, 53, 75-85.
http://dx.doi.org/10.1007/s10725-007-9203-1 - 23. Farooq, M.A., Ali, S., Hameed, A., Ishaque, W., Mahmood, K. and Iqbal, Z. (2013) Alleviation of Cadmium Toxicity by Silicon Is Related to Elevated Photosynthesis, Antioxidant Enzymes; Suppressed Cadmium Uptake and Oxidative Stress in Cotton. Ecotoxicology and Environmental Safety, 96, 242-249.
http://dx.doi.org/10.1016/j.ecoenv.2013.07.006 - 24. Vijayarengan, P. (2012) Changes in Growth and Biochemical Constituents in Rice (Oryza sativa L.) under Arsenic Stress. International Journal Research in Botany, 2, 27-33.
- 25. Ekmekci, Y., Tanyolac, D. and Ayhan, B. (2008) Effects of Cadmium on Antioxidant Enzyme and Photosynthetic Activities in Leaves of Two Maize Cultivars. Journal of Plant Physiology, 165, 600-611.
http://dx.doi.org/10.1016/j.jplph.2007.01.017 - 26. Fujii, H., Hayasaka, T., Yokoyama, K. and Mayum, H. (1999) New Silicon Source for Rice Cultivation; Rooting Ability and Early Growth of Wetland Rice as Affected by Silica Gel Application to the Nursery Bed. Conference “Silicon in Agriculture”, Fort Lauderdale, 26-30 September 1999, 32.
- 27. Perveen, A., Wahid, A. and Javed, F. (2011) Varietal Differences in Spring and Autumn Sown Maize (Zea mays) for Tolerance against Cadmium Toxicity. International Journal of Agriculture and Biology, 13, 909-915.
- 28. Benyas, E., Nassan, A.D.M. and Oustan, S. (2013) Effects of Cadmium on Some Morphological and Physiological traits of Amaranth (Amaranthus caudatus L.) and Oilseed Rape (Brassica napus L.). International Journal of Bioscience, 3, 17-26.
http://dx.doi.org/10.12692/ijb/3.4.17-26 - 29. Shengyi, X., Qishan, W., Xia, S. and Wang, Q.S. (1998) Studies on the Effect of Silicon Fertilizer on Cotton. China Cotton, 25, 6-7.
- 30. Mei, L., Wan, X., Huang, Z., Chen, T., Li, X. and Liu, Y. (2012) First Evidence on Different Transportation Models of Arsenic and Phosphorus in Arsenic Hyperaccumulator Pteris vittata. Journal of Environmental Pollution, 161, 1-7.
http://dx.doi.org/10.1016/j.envpol.2011.09.017 - 31. Shin, H., Shin, H.S., Dewbre, G.R. and Harrison, M.J. (2004) Phosphate Transport in Arabidopsis: Pht1; 1 and Pht1;4 Play a Major Role in Phosphate Acquisition from both Low- and High-Phosphate Environments. The Plant Journal, 39, 629-642.
http://dx.doi.org/10.1111/j.1365-313X.2004.02161.x - 32. Duan, G.L., Zhu, Y.G., Tong, Y.P., Cai, C. and Kneer, R. (2005) Characterization of Arsenate Reductase in the Extract of Roots and Fronds of Chinese Brake Fern, an Arsenic Hyperaccumulator. Journal of Plant Physiology, 138, 461-469.
http://dx.doi.org/10.1104/pp.104.057422 - 33. Xu, Z., Di, T., Zhu, Y., Shen, Q. and Xu, G. (2008) Response of Plasma Membrane H+-ATPase of Rice Roots to the P Deficiency. Journal of Plant Nutrition and Fertilizer Science, 14, 221-226.
NOTES
*Corresponding author.