Modern Research in Inflammation
Vol.05 No.02(2016), Article ID:66351,7 pages
10.4236/mri.2016.52002
Procalcitonin―A Useful Biomarker for Pneumonia Associated with Rhodococcus equi?
Ann Kristin Barton1*, Martin Rieger2, Dana Teschner1, Heidrun Gehlen1
1Equine Clinic, Veterinary Faculty, Freie Universitaet Berlin, Berlin, Germany
2Helmholtz Center, München, Germany

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 16 February 2016; accepted 8 May 2016; published 11 May 2016
ABSTRACT
Background: Procalcitonin, a precursor protein of the hormone calcitonin, is a sensitive marker for sepsis in human medicine, which is used for diagnosis of bacterial pneumonia in adults and neonates to initiate antibiotic therapy. Objectives: In this study, procalcitonin was evaluated as a potential biomarker for Rhodococcus equi associated pneumonia. Methods: In four foals procalcitonin was measured at four time-points (day 0 before antibiotic therapy, day 1, 3 and 5/6 during therapy) in plasma using an equine specific ELISA. Inclusion criteria for the study were a positive sepsis score, ultrasonographic evidence of pulmonary abscesses in addition >8 cm, a positive microbiology out of tracheobronchial secretion and positive response to antibiotic treatment (azithromycin 10 mg/kg BDW 24q PO and rifampicin 10 mg/kg BDW 12q PO) within a weak including improvement of clinical status and reduction of ultrasonographic score <8 cm. Results: Procalcitonin concentrations remained below the working range of the ELISA (25 - 1000 ng/ml) in all but one sample. Conclusions: Procalcitonin cannot be regarded a useful biomarker in pneumonia associated with Rhodococcus equi.
Keywords:
Foal, Horse, Lung, Pneumonia, Procalcitonin, Rhodococcus equi

1. Introduction
Procalcitonin (PCT) is a precursor protein of the hormone calcitonin, which regulates the calcium homeostasis by inhibition of osteoclastic activity. In health, preprocalcitonin (prePCT) is exclusively produced in the thyroid c-cells. Low concentrations of <0.1 ng/ml are found in human serum [1] .
Since the 1970s hypocalcemia has been studied in correlation to sepsis [2] . During sepsis, PCT is found in high concentrations in blood and almost all tissues [3] . It not only is a precursor of calcitonin leading to hypocalcemia, but also seems to have another pathophysiologic role as an inflammatory mediator and is found in almost all inflammatory processes independent from hypocalcemia. As PCT concentrations in plasma rise quickly―within 3 hours after experimental sepsis induction by intravenous endotoxin PCT levels in men increase significantly―PCT has become a useful point-of-care parameter in intensive care units [4] . It has been shown that mortality increases by 7.6% with every hour of delayed treatment [5] .
In horses, and particular in foals, the gastrointestinal tract and the lung are common primarily affected organ systems in sepsis. In neonatal foals, sepsis is one of the main causes of death [6] . Diagnosis of sepsis by blood cultures takes 48 - 72 hours [7] , which is too long to ensure survival in these foals.
Pulmonary inflammation in men is associated with lower PCT concentrations compared to endotoxemia and sepsis, but differentiation between different forms of pneumonia is still possible [8] - [10] . For example, PCT has been used as a biomarker in the diagnosis of tuberculosis [11] .
Rhodococcosis, a pyogranulomatous pneumonia caused by the gram-positive saprophyte Rhodococcus equi, is a pulmonary disease with a high ecomic impact in foals worldwide [12] [13] . Recommended antibiotics, commonly a combination of rifampicin and a macrolide, are expensive drugs and duration of therapy is very long with 4 - 6 weeks, although it could be shown that number and size of abscesses clearly decrease within 1 - 2 weeks under adequate therapy [14] . Regular ultrasonographic controls have been found most useful to check the success of therapy, while different blood parameters were unreliable including the white blood cell count and fibrinogen concentration [15] [16] .
In this study, we aimed to evaluate the biomarker procalcitonin for its usefulness as an early indicator of sepsis and to control treatment success in foals suffering from Rhodococcus equi associated pneumonia. Rieger et al. established a new species specific ELISA for equine PCT, which allows evaluating the actual PCT concentration and not only its gene expression with a high specifity and sensitivity [17] . We figured that this biomarker might allow reducing antibiotic treatment in the long term and shorten the duration of therapy in affected foals.
2. Materials and Methods
During a first disease outbreak of Rhodococcus equi associated pneumonia in a stud, which had been proven by repeated microbiologic examinations of tracheal aspirates, PCT concentrations were measured in 4 foals at different time-points in the course of the disease, beginning before the initiation of antibiotic therapy up to significant clinical improvement.
Overall, more than 20 foals were affected by respiratory disease, but inclusion criteria were strict including a modified positive sepsis score (≥10 points) according to Breuer et al. [18] at the beginning of sampling, an ultrasonographic abscess score >8 cm [14] , positive microbiology for Rhodococcus equi and a first sample before the initiation of antibiotic therapy. In addition, only foals were included into the study, which showed a reduction in sepsis score and ultrasonographic score <8 cm within the first week of therapy.
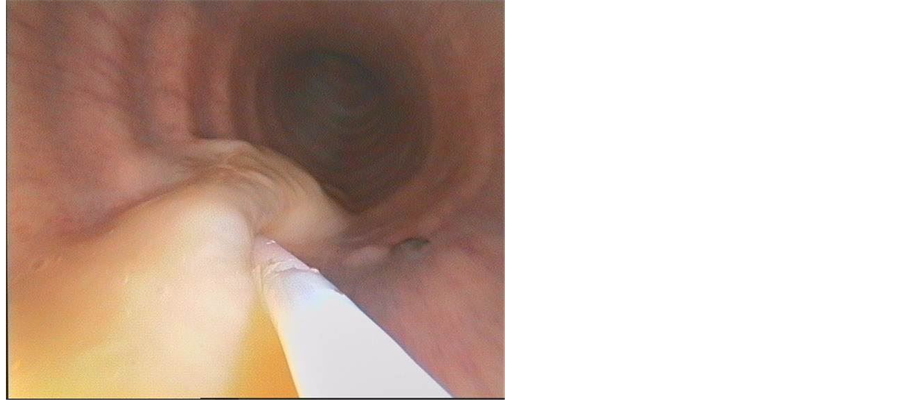
On day 0 clinical, ultrasonographic, endoscopic and laboratory examinations were performed to classify the foals according to a modified sepsis score [18] as shown in Table 1. This score was simplified by reducing clinical pathology to WBC (white blood cells), while the percentage of neutrophils, thrombocyte count and concentration of fibrinogen were not measured. According to the original score results ≥13 out of 41 points are classified as positive for sepsis, but as the maximum score in the modified version was 29 points, we reduced the inclusion border to 10 points for our study. The diameters of abscesses visible in ultrasonography of left and right thorax were added to account for the ultrasonographic score. Endoscopy was performed under sedation (xylazin 0.6 - 1.0 mg/kg BDW IV). In all 4 foals high amounts of viscous mucopurulent secretions were evident in the trachea (Figure 1). Using capped catheter systems tracheal aspirate samples were taken for microbiologic cultures. The jugular vein was punctuated to gain venous blood for clinical pathology (WBC, PCT). Plasma for PCT measurements was frozen at −20˚C and stored on dry ice during transport to the Helmholtz-Center in Munich, where PCT was measured using a species specific ELISA for equine PCT [17] . After first samples were taken, antibiotic therapy including azithromycin (10 mg/kg PO SID) and rifampicin (10 mg/kg PO BID) was initiated.
On day 1 after beginning of antibiotic treatment, on day 3 and after clinical improvement (day 5 - 6) clinical examinations were repeated and further venous blood samples taken for clinical pathology (WBC, PCT). Ultra-
Figure 1. Mucopurulent secretion in the trachea of an affected foal.
Table 1. Sepsis score, modified after Breuer et al. (2012).
sonography was also repeated after clinical improvement.
3. Results
On day 0 all foals presented with lethargy, increased body temperature, dyspnea and leucocytosis. Sonography of the thorax revealed multiple hypoechogenic soft tissue masses interpreted as abscesses adding to a score of 10 - 22 cm. The results of clinical, laboratory and sonographic examinations are shown in Table 2.
Antibiotic therapy was initiated due to former microbiology positive for Rhodococcus equi from tracheal aspirates and pulmonary tissue, before the microbiologic results were available for the individual foal. In less than 50% of affected foals Rhodococcus equi infection was confirmed by microbiology. In six foals cytology and microbiology revealed an infection with Streptococcus equi spp. zooepidemicus (Figure 2 & Figure 3). As this was interpreted as a possible secondary infection, the antibiotic therapy including azithromycin and rifampicin was continued. As already mentioned, only foals were included into the statistical analysis, for which Rhodococcus equi infection was actually proven.
After initiation of antibiotic therapy these 4 foals showed rapid improvement within 5 - 6 days including improvement of general condition and dyspnea. Nevertheless, all but one foal were still febrile. There was also a clear reduction in ultrasonography scores to 4 - 7 cm. In none of the foals a reduction of leukocytosis was evident.
Figure 2. Sonographic appearance of a rough pleural surface, loss of reverberation artefacts, comet tail artefacts and a pulmonary abscess. The sum of abscess diameters accounted for the sonographic score, which was an inclusion criterion >8 cm for the study.
Figure 3. Multiple coccoid bacteria in chains in tracheal aspirate cytology. In several foals Streptococcus equi spp. zooepidemicus was found, probably as secondary infection.
PCT concentrations remained below the working range of the ELISA (25 - 1000 ng/ml) for the whole study period apart from a single measurement (day 0 in foal No. 2).
4. Discussion
Biomarkers are used in human and veterinary medicine for several reasons. They may ease diagnosing disease early, support the decision for or against antibiotic use and to shorten the duration of antibiotic therapy.
In the late 1990s, it has been shown that procalcitonin is a sensitive marker for systemic inflammatory processes caused by bacterial infection, but not of other pathogenesis [19] - [21] . The lung is the primary organ to be affected by Rhodococcus equi, but other organs and joints can be affected as well. Therefore, not only a local reaction, but a systemic inflammatory response is evident in many cases. The high clinical sepsis scores and white blood cell counts support the severity of infection on all 4 foals studied. Equine PCT was characterized in the early 2000s [22] . Hypocalcemia in case of acute systemic disease was also found in horse [23] . Low plasma levels of ionized calcium were found in severe colic, gastrointestinal inflammation and induced endotoxemia
Table 2. Results of clinical and laboratory examinations (sepsis score), thoracic sonography (sonography score) and evaluation of PCT concentration in plasma before (day 0) and under the course of antibiotic therapy (day 1, 3 and 5/6) in 4 foals (No. 1 - 4).
[24] [25] . In foals, in which sepsis is one of the main causes of death in the neonatal period, hypocalcemia has also been correlated to the severity of disease [26] . On the other hand, Pusterla et al. [7] found no significant differences in mRNA expression for IL-1ß, IL-6 and PCT between healthy and septic foals. Nevertheless, the mean PCT expression was higher in septic foals compared to non-septic or healthy foals due to a higher variation of data in sepsis. The authors argued that PCT is not expressed by leucocytes to a high degree, while Toribio [23] described the very complex composition of the CALC-I-gene and questioned the primer sequences in the PCR used. The species specific ELISA used in our study however has been found to have a high sensitivity even in horses, in which sepsis was questionable [17] . In a study on colic patients high concentrations were found using this ELISA as well [27] . Foals may be different, but horses at 3 month of age are not regarded as neonates anymore, and even in neonates, PCT is a valuable biomarker for EONS (early-onset neonatal sepsis) in intensive-care units in human medicine. As early as at 24 h of age PCT is more sensitive as c-reactive protein [28] .
5. Conclusion
In conclusion, procalcitonin cannot be regarded a useful biomarker in pneumonia associated with Rhodococcus equi in the 4 foals of the age group studied. Nevertheless, further studies on a higher number of foals and samples may come to different results.
Cite this paper
Ann Kristin Barton,Martin Rieger,Dana Teschner,Heidrun Gehlen, (2016) Procalcitonin—A Useful Biomarker for Pneumonia Associated with Rhodococcus equi?. Modern Research in Inflammation,05,13-19. doi: 10.4236/mri.2016.52002
References
- 1. Russwurm, S., Oberhoffer, M., Zipfel, P.F., et al. (1999) Procalcitonin—A Novel Biochemical Marker for the Mediator-Directed Therapy of Sepsis. Molecular Medicine Today, 5, 286-287.
http://dx.doi.org/10.1016/S1357-4310(99)01505-1 - 2. Holowaychuk, M.K. and Martin, L.G. (2007) Review of Hypocalcemia in Septic Patients. Journal of Veterinary Emergency and Critical Care, 17, 348-358.
http://dx.doi.org/10.1111/j.1476-4431.2007.00246.x - 3. Müller, B., White, J.C., Nylen, E.S., et al. (2001) Ubiquitous Expression of the Calcitonin-I Gene in Multiple Tissues in Response to Sepsis. The Journal of Clinical Endocrinology & Metabolism, 86, 396-404.
http://dx.doi.org/10.1210/jc.86.1.396 - 4. Preas, H.L., Nylen, E.S., Snider, R.H., et al. (2001) Effects of Anti-Inflammatory Agents on Serum Levels of Calcitonin Precursors during Human Experimental Endotoxemia. The Journal of Infectious Diseases, 184, 373-376.
http://dx.doi.org/10.1086/322031 - 5. Kumar, A., Roberts, D., Wood, K.E., et al. (2006) Duration of Hypotension before Initiation of Effective Antimicrobial Therapy Is the Critical Determinant of Survival in Human Septic Shock. Critical Care Medicine, 34, 1589-1596.
http://dx.doi.org/10.1097/01.CCM.0000217961.75225.E9 - 6. Cohen, N.D. (1994) Causes of and Farm Management Factors Associated with Disease and Death in Foals. Journal of the American Veterinary Medical Association, 204, 1644-1651.
- 7. Pusterla, N., Magdesian, K.G., Mapes, S., et al. (2006) Expression of Molecular Markers in Blood of Neonatal Foals with Sepsis. American Journal of Veterinary Research, 67, 1045-1049.
http://dx.doi.org/10.2460/ajvr.67.6.1045 - 8. Berg, P. and Lindhardt, B.Ø. (2012) The Role of Procalcitonin in Adult Patients with Community-Acquired Pneumonia—A Systematic Review. Danish Medical Journal, 59, A4357.
- 9. Julián-Jiménez, A., Timón Zapata, J., Laserna Mendieta, E.J., et al. (2014) Diagnostic and Prognostic Power of Biomarkers to Improve the Management of Community Acquired Pneumonia in the Emergency Department. Enfermedades Infecciosas y Microbiología Clínica, 32, 225-235.
http://dx.doi.org/10.1016/j.eimc.2013.04.015 - 10. Pereira, J.M., Teixeira-Pinto, A., Basílio, C., et al. (2013) Can We Predict Pneumococcal Bacteremia in Patients with Severe Community-Acquired Pneumonia? Journal of Critical Care, 28, 970-974.
http://dx.doi.org/10.1016/j.jcrc.2013.04.016 - 11. Niu, W.Y., Wan, Y.G., Li, M.Y., Wu, Z.X., Zhang, L.G. and Wang, J.X. (2013) The Diagnostic Value of Serum Procalcitonin, IL-10 and C-Reactive Protein in Community Acquired Pneumonia and Tuberculosis. European Review for Medical and Pharmacological Sciences, 17, 3329-3333.
- 12. Muscatello, G., Anderson, G.A., Gilkerson, J.R. and Browning, G.F. (2006) Associations between the Ecology of Virulent Rhodococcus equi and the Epidemiology of R. equi Pneumonia on Australian Thoroughbred Farms. Applied and Environmental Microbiology, 72, 6152-6160.
http://dx.doi.org/10.1128/AEM.00495-06 - 13. Venner, M., Reinhold, B., Beyerbach, M. and Feige, K. (2009) Efficacy of Azithromycin in Preventing Pulmonary Abscesses in Foals. The Veterinary Journal, 179, 301-303.
http://dx.doi.org/10.1016/j.tvjl.2007.10.002 - 14. Venner, M., Credner, N., Lämmer, M. and Giguère, S. (2013) Comparison of Tulathromycin, Azithromycin and Azithromycin-Rifampin for the Treatment of Mild Pneumonia Associated with Rhodococcus equi. Veterinary Record, 173, 397.
http://dx.doi.org/10.1136/vr.101867 - 15. Giguère, S., Hernandez, J., Gaskin, J., Miller, C. and Bowman, J.L. (2003) Evaluation of White Blood Cell Concentration, Plasma Fibrinogen Concentration, and an Agar Gel Immunodiffusion Test for Early Identification of Foals with Rhodococcus equi Pneumonia. Journal of the American Veterinary Medical Association, 222, 775-781.
http://dx.doi.org/10.2460/javma.2003.222.775 - 16. Venner, M., Astheimer, K., Lämmer, M. and Giguère, S. (2013) Efficacy of Mass Antimicrobial Treatment of Foals with Subclinical Pulmonary Abscesses Associated with Rhodococcus equi. Journal of Veterinary Internal Medicine, 27, 171-176.
http://dx.doi.org/10.1111/jvim.12030 - 17. Rieger, M., Kochleus, C., Teschner, D., et al. (2014) A New ELISA for the Quantification of Equine Procalcitonin in Plasma as Potential Inflammation Biomarker in Horses. Analytical and Bioanalytical Chemistry, 406, 5507-5512.
http://dx.doi.org/10.1007/s00216-014-7944-z - 18. Breuer, J. and Schusser, G.F. (2012) Establishing a Sepsis-Score for Adult Equine Patients. Pferdeheilkunde, 28, 421-428.
- 19. Karzai, W., Oberhoffer, M., Meier-Hellmann, A. and Reinhart, K. (1997) Procalcitonin—A New Indicator of the Systemic Response to Severe Infections. Infection, 25, 329-334.
http://dx.doi.org/10.1007/BF01740811 - 20. Müller, B., Becker, K.L., Schachinger, H., Rickenbacher, P.R., Huber, P.R., Zimmerli, W. and Ritz, R. (2000) Calcitonin Precursors Are Reliable Markers of Sepsis in a Medical Intensive Care Unit. Critical Care Medicine, 28, 977-983.
http://dx.doi.org/10.1097/00003246-200004000-00011 - 21. Russwurm, S., Wiederhold, M., Oberhoffer, M., Stonans, I., Zipfel, P.F. and Reinhart, K. (1999) Molecular Aspects and Natural Source of Procalcitonin. Clinical Chemistry and Laboratory Medicine, 37, 789-797.
http://dx.doi.org/10.1515/CCLM.1999.119 - 22. Toribio, R.E., Kohn, C.W., Leone, G.W., Capen, C.C. and Rosol, T.J. (2003) Molecular Cloning and Expression of Equine Calcitonin, Calcitonin Gene-Related Peptide-I, and Calcitonin Gene-Related Peptide-II. Molecular and Cellular Endocrinology, 199, 119-128.
- 23. Toribio, R.E. (2011) Endocrine Dysregulation in Critically Ill Foals and Horses. Veterinary Clinics of North America: Equine Practice, 27, 35-47.
http://dx.doi.org/10.1016/j.cveq.2010.12.011 - 24. Garcia-Lopez, J.M., Provost, P.J., Rush, J.E., Zicker, S.C., Burmaster, H. and Freeman, L.M. (2001) Prevalence and Prognostic Importance of Hypomagnesemia and Hypocalcemia in Horses That Have Colic Surgery. American Journal of Veterinary Research, 62, 7-12.
http://dx.doi.org/10.2460/ajvr.2001.62.7 - 25. Toribio, R.E., Kohn, C.W., Hardy, J. and Rosol, T.J. (2005) Alterations in Serum Parathyroid Hormone and Electrolyte Concentrations and Urinary Excretion of Electrolytes in Horses with Induced Endotoxemia. Journal of Veterinary Internal Medicine, 19, 223-231.
http://dx.doi.org/10.1111/j.1939-1676.2005.tb02686.x - 26. Hurcombe, S.D.A., Toribio, R.E., Slovis, N.M., Saville, W.J., Mudge, M.C., MacGillivray, K. and Frazer, M.L. (2009) Calcium Regulating Hormones and Serum Calcium and Magnesium Concentrations in Septic and Critically Ill Foals and Their Association with Survival. Journal of Veterinary Internal Medicine, 23, 335-343.
http://dx.doi.org/10.1111/j.1939-1676.2009.0275.x - 27. Teschner, D., Rieger, M., Koopmann, C. and Gehlen, H. (2015) Procalcitonin bei Pferden mit akuter Kolik. Pferdeheilkunde, 31, 317-377.
- 28. Altunhan, H., Annagür, A., Örs, R. and Mehmetoglu, I. (2011) Procalcitonin Measurement at 24 Hours of Age May Be Helpful in the Prompt Diagnosis of Early-Onset Neonatal Sepsis. International Journal of Infectious Diseases, 15, 854-858.
http://dx.doi.org/10.1016/j.ijid.2011.09.007
NOTES
*Corresponding author.