New Journal of Glass and Ceramics
Vol.3 No.3(2013), Article ID:34146,6 pages DOI:10.4236/njgc.2013.33013
Effects of Co2O3 on Crystallization and Colorization of Lithium Aluminosilicate Glass Ceramics
![]()
The Department of Materials Science and Engineering, Zhejiang University, Hangzhou, China.
Email: *yanghui@zju.edu.cn
Copyright © 2013 Xingzhong Guo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 23rd, 2013; revised February 22nd, 2013; accepted March 9th, 2013
Keywords: Glass Ceramic; Crystal Size; Crystallization; Microstructure
ABSTRACT
The effects of Co2O3 on the crystallization and colorization of lithium aluminosilicate glass ceramic were investigated by using differential thermal analysis (DTA), X-ray diffractometry (XRD) and scanning electron microscope (SEM). The results showed that the introduction of Co2O3 not only changed the color of lithium aluminosilicate glass but also affected its crystallization by increasing the crystallizing maximum peak temperature (Tp) and weakening the crystallization ability. In addition, the color of LAS glass ceramics could be achieved by controlling the suitable Co2O3 and appropriate crystallization temperature.
1. Introduction
The lithium aluminosilicate (Li2O-Al2O3-SiO2, LAS) system glass ceramic has been widely studied because of its low expansion, high heat resistance, outstanding chemical durability and excellent mechanical properties [1-5]. The colorization of LAS glass ceramics has become a hotspot in this field. The coloring process of glass ceramic is not only related to the system of glass ceramics, but also depends on the composition of coloring agent and heat treatment process. There are not too many studies to investigate the effects of single Co2O3 on crystallization, structure and performances of lithium aluminosilicate glass ceramics.
Based on our previous studies on the nucleation and crystallization of LAS glass [6-9], this paper aims to investigate the effect of single Co2O3 on the crystallization and colorization of LAS glass ceramics by employing DTA, XRD and SEM.
2. Experimental
2.1. Glass preparation
Acid washed quartz sands, Li2CO3, Al2O3, MgO, ZnO, TiO2 and other compositions were used to produce the glass batch, whose proportions were listed in Table 1. The different ratios of Co2O3 were added into the glass batch and employed as a coloring agent. The raw materials all together were melted for 4 - 6 h at 1673 - 1723 K and moulded in a pre-heated die. The glass samples were then annealed at 773 K for 1 h to eliminate internal stress [4-6].
2.2. Nucleation and Crystallization
The heat-treatment of the annealed glass samples was carried out at 873 K, 893 K, 993 K, 1093 K and 1193 K for 1 h with a heating rate of 20 K·min−1, respectively. Subsequently, the samples were fast-cooled, ground and sieved through a 200-mesh screen to obtain the glass powder.
Differential thermal analysis (DTA) of the annealed glass samples was carried out on a differential thermal

Table 1. Main compositions of the glass batches (wt%).
analyzer (NETZSCH STA 409 PC Luxx, Germany) with alumina as the reference and the samples were heated at 5-20 K·min−1 from 293 K to 1573 K. The crystalline phases of the samples were analyzed by the X-ray diffraction (XRD) method on a XJ 10-60 X-ray diffractometer using nickel filtered Cu Kα radiation in the range of 2θ = 10˚ - 80˚ with a scanning speed of 2˚/min. The surface of the samples was polished and eroded by HF (2 wt%) for 30 - 40 s for the further morphology observation on the scanning electron microscopy (FEI SIRION).
3. Results and Discussions
3.1. Crystallization
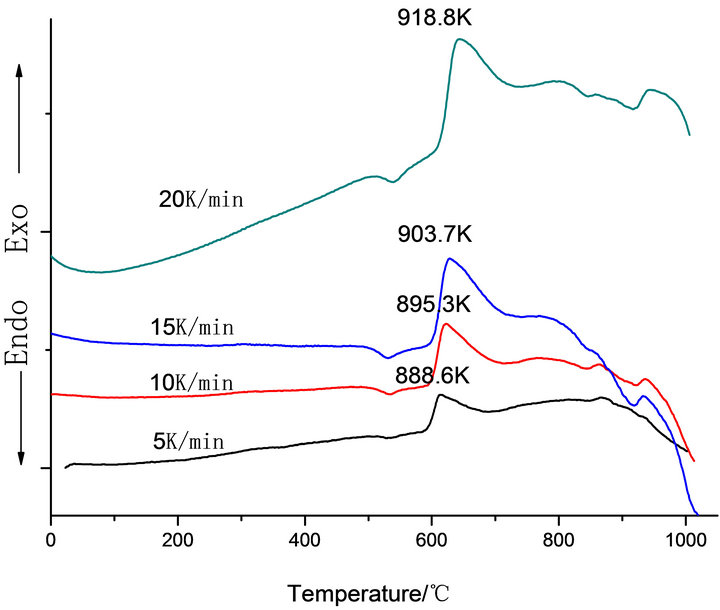
Figure 1 shows the DTA curves obtained from as-cast LAS glass and Table 2 shows the crystallizing peak maximum temperatures (Tp) from DTA curves at different heating rates. According to curves, it is shown that crystallizing maximum peak temperature (Tp) increases with the increase of heating rates and Co2O3 contents, respectively.
The crystallization kinetic characteristics of lithium aluminosilicate glass can be decided as follows by Arrhenius [11], Kissinger [12] and Augis-Bennett [13], which are respectively expressed as
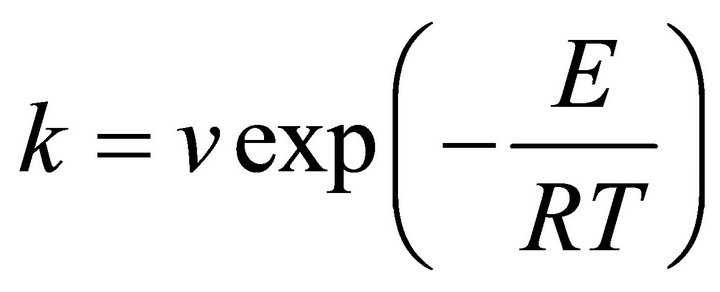 (1)
(1)
 (2)
(2)
 (3)
(3)
where in E is the activation energy (kJ·mol−1), R the gas constant, ν the frequency factor, a the DTA heating rate
 (a)
(a) (b)
(b) (c)
(c) (d)
(d)
Figure 1. DTA traces obtained from: (a) G-0; (b) G-1, (c) G-2 and (d) G-3 LAS glass powders.

Table 2. Tp (K) values from DTA curve of LAS glass samples at different heating rates.

Figure 2. Relationship between ln( ) and 1/Tp: G-0, G-1, G-2, G-3.
) and 1/Tp: G-0, G-1, G-2, G-3.
(K/min), k the reaction rate constant, which is related to the E and ν, n the crystallization index, i.e. Avrami exponent, depending upon the morphology or directionality of crystal growth and  is the half-height temperature wideness of the maximum exothermical peak of DTA. According to Equations (1)-(3), low E value and high ν lead to high k, indicating high crystallization rate and crystallinity. Crystallization index n is related to crystallization manner, n ≈ 1, surface crystallization, n ≈ 2 twodimension growth crystallization and n ≈ 3, volumetric crystallization [8].
is the half-height temperature wideness of the maximum exothermical peak of DTA. According to Equations (1)-(3), low E value and high ν lead to high k, indicating high crystallization rate and crystallinity. Crystallization index n is related to crystallization manner, n ≈ 1, surface crystallization, n ≈ 2 twodimension growth crystallization and n ≈ 3, volumetric crystallization [8].
The relationship between  and
and  is constructed (Figure 2) to calculate the effective activation energy, frequency factor and crystallization index, as shown in Table 3. The G-1 specimen has the highest E and v than other specimens. The G-2 and G-3 have lower E and v than G-0. It is suggested that the G-1 (containing Co2O3 0.001% content) can increase the activation energy and frequency factor, but the k value becomes lower than G-0 in the end. However, the G-2 and G-3 can lower the activation energy and frequency factor, their k values become lower too. In other words, comparing to G-0, the ability of crystallizations would be weakened with the increase of Co2O3 content.
is constructed (Figure 2) to calculate the effective activation energy, frequency factor and crystallization index, as shown in Table 3. The G-1 specimen has the highest E and v than other specimens. The G-2 and G-3 have lower E and v than G-0. It is suggested that the G-1 (containing Co2O3 0.001% content) can increase the activation energy and frequency factor, but the k value becomes lower than G-0 in the end. However, the G-2 and G-3 can lower the activation energy and frequency factor, their k values become lower too. In other words, comparing to G-0, the ability of crystallizations would be weakened with the increase of Co2O3 content.
The n values, which are calculated by using Equation (3), are given in the Table 3. The fact n value is near 1 indicates that crystallization manner of LAS glass is surface crystallization, while n value near 2 suggests two-dimensional growth crystallization. From Table 3, it can be seen that Co2O3 can change the crystallization manner of glass.

Table 3. E, v, and n crystallization values of the LAS glass samples.
 (a)
(a) (b)
(b)
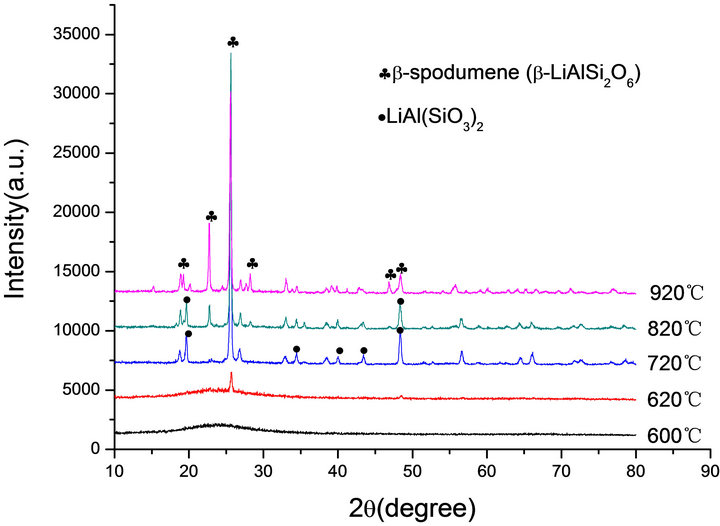
Figure 3. XRD patterns of: (a) G-0 and (b) G-3 LAS glass heat treated at different crystallization temperatures.

Figure 4. XRD patterns of G-0, G-1, G-2 and G-3 LAS glass heat treated at 1093 K.
The crystallization peaks on the DTA curves imply that crystal phase forms and transforms during the heat treatment. Figure 3 shows the diffraction patterns of G-0 and G-3 samples. Both of G-0 and G-3 glass samples are amorphous phase below 893 K. There is only hexagonal LiAl (SiO3)2 crystal phase (JCPDS-PDF 31-0706) in the temperature range of 993 K to 1093 K, which is similar to α-spodumene. The β-spodumene (JCPDS-PDF 35-07 97) was main crystallization phase in both samples at 1093 K. In addition, the specimen became opaque due to the crystallinity. It is also confirmed that, above 1093 K, the transformation of α-spodumene into β-spodumene finished.
According to the XRD patterns of G-0, G-1, G-2 and G-3 LAS glass samples heat treated at 1093 K (Figure 4), G-0, G-2 and G-3 have the formation of main crystallization phase of β-LiAlSi2O6, but we can find only LiAl (SiO3)2 in the G-1. It indicates that the content of 0.001% Co2O3 can restrain the main phase transformation of α-spodumene into β-spodumene at 1093 K.
Figure 5 shows the microstructures of G-0 and G-3 samples heat treated for 1 h at different crystallization temperatures. It can be seen that the grain size of both G-0 and G-3 samples increases with the crystallization temperatures, and the grain size of G-0 specimen is similar to that of the G-3 specimen. The G-3 crystal grain becomes from sphere-shape to needle. And both of specimen still exist amorphous phase below 993 K, crystal phase grain is about 20 - 30 nm. According to Figure 5 (c)-(j), it is found that Co can slow crystal growth rates, which indicates that the addition of Co has no significant effect on the grain size, but it can control crystal growth rate of LAS glass ceramics. This was certificated by analyzing Table 3.
Figure 6 shows the microstructures of G-0, G-1, G-2 and G-3 samples heat treated for 1 h at 1093 K. The G-0, G-1 and G-2 specimens have similar complete structures;
 (a)
(a) (b)
(b) (c)
(c) (d)
(d) (e)
(e) (f)
(f) (g)
(g) (h)
(h) (i)
(i) (j)
(j)
Figure 5. SEM images of G-0 and G-3 glass samples heat treated for 1 h at different crystallization temperatures.
 (a)
(a) (b)
(b) (c)
(c) (d)
(d)
Figure 6. SEM images of G-0, G-1, G-2 and G-3 glass samples heat treated for 1 h at 1193 K.
however, the G-3 has obviously different shape. It shows if the content of Co (containing 0.1% Co2O3) is high, it will have remarkable effect on the crystal grain shapes in that Co can capture ion O2− (non bridge oxygen) which results in incomplete glass structure.
3.2. Colorization
Figure 7 shows the color of glass ceramic samples at different crystallization temperatures. Compared G-0 with G-1, it is shown that little Co (lower than 0.001% Co2O3) have hardly effect on the color of glass. With the increase of the contents of Co, all glass samples’ color become from transparent to blue or navy blue(even near to black) at below 893 K. The color of G-2 and G-3 become light with the increase of the crystallization temperature. According to Figures 3 and 5, crystal phases have formed above 993 K. So we can obtain different color glass-ceramics by controlling the suitable contents of Co and appropriate crystallization temperature.
Co3+ ion is known to absorb visible light wavelengths and used to colorize glass. The colorization Co3+ ions in the structure of glass are surrounded by O2− ions to form different coordination ligands and states, which play an essential part in the glass structure and spectrum characteristics. Co3+ ions can form four or six ligands and join the silicon oxygen tetrahedron together. With radii of 0.065 nm, Co3+ ion is strong in terms of electric field intensity, which results in the high capacity of joining the silicon oxygen tetrahedrons and reducing the crystallization ability of LAS glass.
4. Conclusions
The crystallization mechanism and microstructure of Li2O-Al2O3-SiO2 system glass ceramic containing Co2O3 was investigated by employing DTA, XRD and SEM. The introduction of Co2O3 increased the crystallization temperature of LAS glass, decreased the crystallization kinetic parameters and obtained the crystalline phases of β-spodumene. The color of LAS glass ceramics could be obtained by controlling the suitable contents of Co2O3

Figure 7. Color of glass ceramic samples at different crystallization temperatures.
and appropriate crystallization temperature. When the content of coloring agent Co2O3 was lower than 0.001%, it had no effect on crystal structure and colorization while it could restrain the main phase transformation of α-spodumene into β-spodumene at 1193 K.
5. Acknowledgements
This work is supported by the Key Technologies R & D Program of China (NO. 2013BAE03B01).
REFERENCES
- P. F. James, “Glass Ceramics: New Compositions and Uses,” Journal of Non-Crystalline Solids, Vol. 181, No. 1-2, 1995, pp. 1-15. doi:10.1016/0022-3093(94)00515-X
- P. A. Tick, N. F. Borrelli and I. M. Reaney, “The Relationship between Structure and Transparency in GlassCeramic Materials,” Optical Materials, Vol. 15, No. 1, 2000, pp. 81-91. doi:10.1016/S0925-3467(00)00017-3
- M. F. Zawrah and E. Hamzawy, “Effect of Cristobalite Formation on Sinterability, Microstructure and Properties of Glass/Ceramic Composites,” Ceramics International, Vol. 28, No. 2, 2002, pp. 123-130. doi:10.1016/S0272-8842(01)00067-0
- X. Guo, H. Yang and M. Cao, “Nucleation and Crystallization Behavior of Li2O-Al2O3-SiO2 System Glass-Ceramic Containing Little Fluorine and No-Fluorine,” Journal of Non-Crystalline Solids, Vol. 351, No. 24-26, 2005, pp. 2133-2137. doi:10.1016/j.jnoncrysol.2005.04.071
- X. Guo and H. Yang, “Effects of Fluorine on Crystallization, Structure and Performances of Lithium Aluminosilcate Glass Ceramic,” Materials Research Bulletin, Vol. 41, No. 2, 2006, pp. 396-405. doi:10.1016/j.materresbull.2005.08.002
- H. Yang and X. Z. Guo, “Effect of Colouring Agent on Colourisation and Structure of Li2O-Al2O3-SiO2 Glass Ceramics,” International Journal of Materials and Product Technology, Vol. 37, No. 3-4, 2010, p. 280. doi:10.1504/IJMPT.2010.031427
- X. Guo, H. Yang, C. Han and F. Song, “Nucleation of Lithium Aluminosilicate Glass Containing Complex Nucleation Agent,” Ceramics International, Vol. 33, No. 7, 2007, pp. 1375-1379. doi:10.1016/j.ceramint.2006.04.019
- X. Guo, H. Yang, C. Han and F. Song, “Crystallization and Microstructure of Li2O-Al2O3-SiO2 Glass Containing Complex Nucleating Agent,” Thermochim Acta, Vol. 444, No. 2, 2006, pp. 201-205. doi:10.1016/j.tca.2006.02.016
- X. Z. Guo, H. Yang, M. Cao, C. Han and F. F. Song, “Crystallinity and Crystallization Mechanism of Lithium Aluminosilicate Glass by X-Ray Diffractometry,” Transactions of Nonferrous Metals Society of China, Vol. 16, No. 3, 2006, pp. 593-597. doi:10.1016/S1003-6326(06)60104-0.
- X.-Z. Guog, H. Yang, Z. Shen, M.-X. Chen and Y.-H. Xiang, “Crystallization Mechanism of Lithium Aluminosilcate Glass Containing Co, Ni and Fe,” The Chinese Journal of Nonferrous Metals, Vol. 17, 2007, pp. 903- 908.
- S. Yilmaz, O. T. Ozkan and V. Gunay, “Crystallization Kinetics of Basalt Glass,” Ceramics International, Vol. 22, No. 6, 1996, pp. 477-481. doi:10.1016/0272-8842(95)00118-2
- H. E. Kissinger, “Variation of Peak Temperature with Heating Rate,” Journal of Research of the National Bureau of Standards, Vol. 57, No. 4, 1956, pp. 217-222. doi:10.6028/jres.057.026
- J. A. Augis and J. E. Bennett, “Calculation of the Avrami Parameters for Heterogeneous Solid State Reactions Using a Modification of the Kissinger Method,” Journal of Thermal Analysis, Vol. 13, No. 2, 1978, pp. 283-292. doi:10.1007/BF01912301
NOTES
*Corresponding author.

