International Journal of Organic Chemistry
Vol.07 No.04(2017), Article ID:79873,11 pages
10.4236/ijoc.2017.74024
Synthesis and Extraction Studies of Calix[4]-Crown-4 Oxime Derivatives
Gülderen Uysal Akkuş1*, Selma Aslan2
1Department of Chemistry, Faculty of Art and Science, Afyon Kocatepe University, Afyonkarahisar, Turkey
2Université d’Aix-Marseille-Faculté des Sciences-Site Etoile 52, Marseille, France
Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: August 24, 2017; Accepted: October 23, 2017; Published: October 26, 2017
ABSTRACT
The article comprises synthesis of calyx[4]-oxa-crown, and calix[4]-thia-crown compounds containing nitrile groups (3a, 3b) and amino groups (4a, 4b) and their corresponding oxime derivatives (5a, 5b) and liquid-liquid extraction studies of these compounds. The oxime derivatives of compounds (5a, 5b) have been synthesized by reacting of di-n-butylamino derivatives of calix[4]-oxa-crown, and calix[4]-thia-crown compounds (4a, 4b) with amphi-chloroglyoxime in methanol-THF. Their cation and anion transfer studies were performed by using liquid-liquid extraction procedure. It has been concluded from the observations that the compound 3a shows a good extraction behavior toward Na+ ion in the presence of other metal cations. Whereas, its oxime derivatives transfers all of the metal cations used in the liquid-liquid extraction studies.
Keywords:
Calix[4]Arene, Calixcrown, Calix-Oxime, Ion Exchange, Liquid-Liquid Extraction

1. Introduction
With the development of technology, environmental pollution has become an important problem today. In order to solve this problem, supramolecular chemistry develops new synthetic methods and separation techniques [1] [2] [3] [4] . In recent years, a number of studies have been carried out on the synthesis of new macrocyclic ligands that can be complex with toxic metals and anions, which play an important role in environmental contamination [5] [6] [7] . Previous studies on supramolecular chemistry have focused on two-dimensional complexes of neutral ionophores such as crown ethers and cryptands with some ionic molecules. In recent years, it is known that calixarenes, a phenol-formaldehyde oligomer, have a cyclic structure, such as in crown ethers, making complexes with cations, anions, and neutral molecules [8] [9] [10] [11] . Recently, new studies have been carried out to find the crown ring in the calix skeleton to combine the unique properties of both lines into a single molecule [12] [13] [14] . These compounds, called calixcrown, are formed by linking phenolic oxygens with poly (oxy) ethylene units in the molecule [1] . The first member, calixcrown compound was reported as crown ethers are combined through the bridging of the phenolic oxygen atoms of the calixarene early as 1983 and in this connection various calix[4]crowns have been reported with their host-guest properties in metal complexation, metal extraction, metal transportation, molecular switches and in ion sensing devices [12] [13] [14] [15] . Due to their highly selective metal ion recognition, which depends on the crown size, on the macrocyclic conformation (especially for calix[4]arene derivatives) and on the substituents at the upper or lower rims, the design and synthesis of these molecules have been very developed in the last few years [16] - [21] .
These compounds form complexes with alkali metals [22] [23] , especially cesium [24] [25] [26] are promising in the nuclear waste management, with heavy metal ions [27] [28] are useful in view of environmental protection and with lanthanides and actinides are used for their removal from industrial and nuclear waste [29] .
The main aim of this study is to synthesize oxime derivatives of calix-crown compounds and to study their ion transport properties. Because the studies which related oxime derivatized calixarenes are restricted numbers [30] , [31] , we decided to synthesis of oxime derivatives of 4a and 4b compounds. We thought that the extraction properties of these compounds would be enhanced if we combine two properties, one of which is crown rings and the other one is oxime groups with calixarene skeleton. Because these compounds have different cavity sizes and different donor atoms, which make them potential hosts for the complexation with metal ions, neutral guests and the formation of charge transfer complexes.
2. Experimental
2.1. Apparatus
Melting points were determined on a Barnsted/Electro thermal apparatus in a sealed capillary and were uncorrected. 1H NMR spectra were recorded on a Bruke Avance DPX 400 spectrometer in CDCl3 with TMS as an internal standard. IR spectra were recorded on a Perkin-Elmer 1605 FTIR System Spectrum BX spectrometer as KBr pellets. UV-vis spectra were obtained on a Shimadzu UV-1700 Pharma visible recording spectrophotometer. Elemental Analysis was recorded on an Elemental CHNS.
2.2. Materials
Analytical TLC were performed on precoated silica gel plates (SiO2, Merck PF254), while silica gel 60 (Merck, particle, size 0.040 - 0.063 mm, 230 - 240 mesh) was used for preparative column chromatography. The drying agent employed was anhydrous sodium sulfate. All aqueous solutions were prepared with deionized water that had been passed through a Milli-Q Plus water purification system.
2.3. Synthesis
Compounds 1, 2a and 2b were synthesized according to the procedures described in the literature [32] [33] . The other compounds 3a-5b employed in this work as illustrated in Scheme 1, have been synthesized as described below:
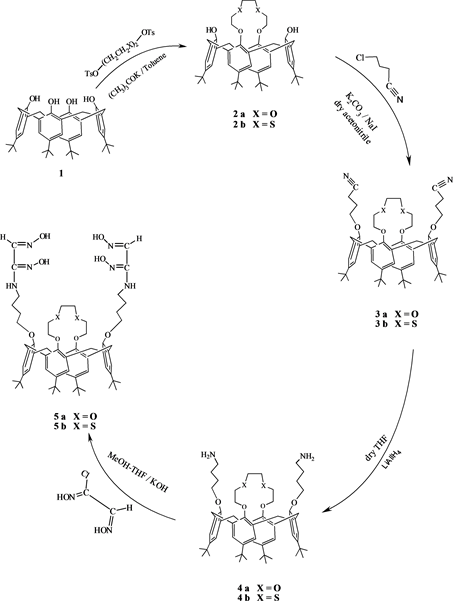
Scheme 1. Synthesis of compound 5a-5b.
2.3.1. 5,11,17,23-Tetra-Tert-Butyl-25,27-Bis(3-Cyanopropoxy) Calix[4]-Oxacrown-4 (3a)
A mixture of compound 2a (4.00 g, 5.10 mmol), K2CO3 (2.81 g, 20.30 mmol), sodium iodide (3.00 g, 20.0 mmol) and 4-chlorobutyronitrile (4.0 g, 20.40 mmol) in dry acetonitrile (200 mL) was stirred and heated under reflux for 24 hr. The solvent was removed in vacuo and 50 ml 2N HCl and 50 ml CH2Cl2 were added and the phases were separated. The aqueous phase was extracted two times with 30 ml CH2Cl2, the organic phases were combined dried with Na2SO4 and the solvent removed in vacuo. The crude product was recrystallized from methanol/CH2Cl2 (5:1). Yield: 44%, mp: 187˚C, IR (KBr) cm−1: 2248 cm−1 (CN). 1H NMR (CDCl3), 1.02 - 1.40 (brs, 36H, But), 1.87 - 2.01 (m, 4H, C-CH2-C), 2.23 (t, 4H, J = 7 Hz, OCH2CH2), 3.28 (t, 4H, J = 7 Hz, CH2CN), 3.37 (d, 4H, J = 13 Hz, ArCH2Ar ), 3.71 - 4.2 (brm, 12H, CH2O), 4.28 (d, 4H, J = 12 Hz, ArCH2Ar), 6.90 - 7.15 (m, 8H, ArH). Calculated for C58H76N2O6, C, 77.67; H, 8.53; N, 3.12. Found: C, 77.50; H, 8.48; N, 3.07.
2.3.2. 5,11,17,23-Tetra-Tert-Butyl-25,27-Bis(3-Cyanopropoxy) Calix[4]-Thiacrown-4 (3b)
A mixture of compound 2b (3.5 g, 4.40 mmol), K2CO3 (5.73 g, 17.6 mmol), sodium iodide (2.63, 17.6 mmol) and 4-chlorobutyronitrile (1.82 g, 17.6 mmol) in dry acetonitrile (175 mL) was stirred and heated under reflux for 24 hr. The reaction procedure was then proceeded according to the above described method. Usual work up afforded 3b. Yield: 54%, mp: 147˚C. IR (KBr) cm−1: 2225 cm−1 (CN). 1H NMR (CDCl3), 1.00 - 1.41 (brs, 36H, But), 1.90 - 1.99 (m, 4H, CH2CH2-CH2), 2.34 (t, 8H, J = 6 Hz, OCH2CH2), 3.21 (t, 4H, J = 7 Hz, CH2CN), 3.27 (d, 4H, J = 8 Hz, ArCH2Ar), 3.34 - 3.39 (m, 8H, CH2S), 4.17 (d, 4H, J = 13 Hz, Ar-CH2Ar), 6.82 - 7.30 (m, 8H, ArH), Calculated for C58H76N2O4S2, C, 74.95; H, 8.24; N, 3.01; S, 6.90. Found: C, 74.63; H, 8.16; N, 2.98.
2.3.3. 5,11,17,23-Tetra-Tert-Butyl-25,27-Bis(4-Aminobutyoxy) Calix[4]-Oxacrown-4 (4a)
Dry THF (50 mL) was added in a two necked glass flask then LiAlH4 (0.27 g, 7.26 mmol 1:2 equation) was added carefully. The reaction mixture was heated until the boiling point of the solvent. A mixture of compound 3a (3.84 g, 3.63 mmol) in the warm dry THF (100 mL) was added dropwise over a period of 1.5 h. and reflux was continued for an additional 7 h. At the end of this time in order to removing the LiAlH4 remaining from the reaction, distilled water (approximately 5 mL) was added slowly little by little in the ice cold bath until the hydrogen gas emission ended. After the removal of most of the solvent it was taken in the separated funnel with CHCl3 then pH was regulated at 4 - 5 with solution of H2SO4 (20 %), and extracted several times with CHCl3. The combined organic layers were finally washed with distilled water, dried over MgSO4, then evaporated to dryness. The residue was recrystallization was performed in ethanol. Yield: 66%, mp: 152˚C. IR (KBr) cm−1: 3400 cm−1 (NH2), 1H NMR (CDC13), δ 1.02 - 1.40 (brs, 36H, But), 1.78 - 2.10 (m, 8H, CH2CH2), 2.49 (t, 4H, J = 8 Hz, OCH2CH2), 2.98 (t, 4H, J = 8 Hz, CH2N), 3.36 (d, 4H, J = 7 Hz, ArCH2Ar), 3.68 - 4.17 (brm, 12H, CH2O), 4.32 (d, 4H, J = 12 Hz, ArCH2Ar ), 5.01 (s, 4H, NH2 ), 6.87 - 7.12 (m, 8H, ArH). Calculated for: C58H84N2O6, C, 76.99; H, 9.35; N, 3.09. Found: C, 76.58; H, 9.02; N, 3.05.
2.3.4. 5,11,17,23-Tetra-Tert-Butyl-25,27-Bis(4-Aminobutyoxy) Calix[4]-Thiacrown-4 (4b)
Dry THF (50 mL) was added in a two necked glass flask then LiAlH4 (0.28 g, 7.30 mmol 1:2 equation) was added carefully. The reaction mixture was heated until the boiling point of the solvent. A mixture of compound 3b (3.95 g, 3.65 mmol) in the warm dry THF (100 mL) was added dropwise over a period of 1.5 h. and reflux was continued for an additional 7 h. The reaction procedure was then proceeded according to the described above. Usual work up afforded 4b. Yield: 64%, mp: 148˚C. IR (KBr ) cm−1: 3412 cm−1 (NH2), 1H NMR (CDC13), δ 1.19 - 1.32 (brs, 36H, But), 1.8 - 2.17 (m, 8H, CH2CH2), 2.49 (t, 4H, J = 7 Hz, OCH2CH2), 3.01 (t, 4H, J = 7 Hz, CH2N), 3.39 (d, 4H, J = 7 Hz, Ar-CH2-Ar), 3.54 - 3.60 (m, 8H, CH2S, CH2O), 3.72 (t, 4H, J = 7 Hz, CH2S), 4.34 (d, 4H, J = 13 Hz, Ar-CH2-Ar), 4.89 (s, 4H, NH2), 6.92 - 7.11 (m, 8H, ArH). Calculated for: C58H84O4N2S2, C, 74.31; H, 9.03; N, 2.98; S, 6.82. Found: C, 74.27; H, 8.92; N, 2.85; S, 6.70.
Synthesis of oxime derivative of compound 4a (5a)
To a solution of compound 4a (4.20 g, 5.0 mmol) in methanol-THF (1:4, 50 mL) was added a solution of amphi-monochloro glyoxime (0.12 g, 10.0 mmol) in MeOH. Then a solution of KOH (% 1 MeOH) was added until pH of the reaction medium is 5. The reaction mixture was stirred at room temperature for 12 h and the solvent was evaporated in vacuo and extracted several times with diethyl ether and dried over Na2SO4. Evaporation of the solvent in vacuo gave the crude product (5a) after recrystallization from EtOH. Yield: 49%, mp: 137˚C. IR (KBr) cm−1, 3200 (OH); 1650 (CN). 1H NMR (CDC13): δ 0.72 - 0.99 (brs, 36H, But), 1.2 - 1.45 (m, 8H, CH2CH2), 2.83 (t, 16H, OCH2CH2), 3.20 (d, 4H, J = 12 Hz, Ar-CH2-Ar), 3.8 - 4.4 (brm, 10H, CH2N, Ar-CH2-Ar, CH=N), 6.37 - 6.41 (m, 10H, ArH, NH), 9.0 (s, 4H, OH). Calculated for: C62H88O10N6 , C, 69.11; H, 8.23; N, 7.80. Found: C, 68.05, H, 8.14; N, 7.75.
Synthesis of oxime derivative of compound 4b (5b)
To a solution of compound 4b (3.5 g, 3.73 mmol) in methanol-THF (1:4, 50 mL) was added a solution of amphi-monochloroglyoxime (0.92 g, 7.46 mmol) in MeOH. Then a solution of KOH (1% MeOH) was added until pH of the reaction medium is 5. The reaction mixture was stirred at room temperature for 12 h. The reaction procedure was then proceeded according to the above described method. Usual work up afforded 5b. Yield: 52%, mp: 151˚C, IR (KBr ) cm−1, 3310 (OH); 1656 (CN). 1H NMR (CDC13): δ 0.91 - 1.41 (brs, 36H, But), 2.71 - 4.38 (brm, 36H, CH2CH2, OCH2CH2, CH2N, Ar-CH2-Ar, CH2S, Ar-CH2-Ar, CH2S, CH2O), 6.94 - 7.20 (m, 12H, ArH, NH, CH=N), 8.95 (s, 4H, OH). Calculated for: C62H88O8N6S2, C, 67.11; H, 7.99; N, 7.58; S, 5.78. Found: C, 67.07, H, 7.84; N, 7.50; S, 5.69.
2.4. Liquid-Liquid Extraction Procedures
Picrate extraction experiments were performed following Pedersen’s procedure [34] A 10 mL of 2.5 × 10−5 M aqueous picrate and 10 mL of 1 × 10−3 M solution of (2a, 3a, 4a, 5a, 2b, 3b, 4b, and 5b) in CH2Cl2 were vigorously agitated in a stoppered glass tube with a mechanical shaker for 2 min. The two-phase systems were then magnetically stirred in a thermostated water-bath at 25˚C for 1 h, and finally left standing for an additional 30 min. The concentration of picrate ion remaining in the aqueous phase was then determined spectrophotometrically. Blank experiments showed that no picrate extraction occurred in the absence of calixarene.
The alkali picrates were prepared as described elsewhere [35] by stepwise addition of a 2.5 × 10−2 M aqueous picric acid solution to a 0.14 M aqueous solution of metal hydroxide, until neutralization which was checked by pH control with a glass electrode. They were then rapidly washed with ethanol and ether before being dried in vacuo for 24 h. Transition metal picrates were prepared by stepwise addition of a 1 × 10−2 M of metal nitrate solution to a 2.5 × 10−5 M aqueous picric acid solution and shaken at 25˚C for 1 h.
The percent extraction (E%) has been calculated as:
(1)
where C0 and C are the initial and final concentrations of the metal picrate before and after the extraction, respectively.
3. Result and Discussion
The properties of calixarenes have been increased in the host-quest chemistry. Because these compounds transport cations, anions and neutral quests selectively. This selectivity is enhanced by functionalizing these compounds with some functional groups. Especially the selectivity is shown when the calix-crown compounds are used. As we know, oxime compounds are complex with metal cations. In this study, our aim is to enhance this present selectivity, so we have joined oxime groups with the calixcrown skeleton. To achieve the desired goal, we have synthesized p-tert-butylcalix[4]arene 1 as well as compounds 2a and 2b as indicated in Scheme 1 according to the previously published procedures [32] , [33] . The compounds 3a and 3b were synthesized by refluxing 2a or 2b with 4-chlorobutyronitrile in the presence of K2CO3/NaI in dry acetonitrile. After purification by reprecipitation from a methanol/CH2Cl2, they were obtained in 44% and 54% yield, respectively. IR spectra showed a nitrile band at 2248 and 2225 cm−1 for 3a and 3b respectively. The 1H NMR spectrum of the compounds 3a and 3b have a typical AB pattern for the methylene bridge protons (ArCH2Ar) of the calixarene moiety at 3.37 and 4.28 ppm (J = 13 Hz), 3.27 and 4.17 (J = 13 Hz), for 3a and 3b respectively, indicating that both of the compounds exist in cone conformation.
Synthesis of the compounds 4a and 4b were fulfilled in 66% and 64% yield respectively by the reduction of nitrile groups of 3a and 3b with LiAlH4 in dry THF. Completion of this reaction was followed by the IR spectroscopy indicating the disappearance of the band due to the nitrile groups at 2248 and 2225 cm−1 for 3a and 3b respectively and the appearance of a new band at 3400 and 3412 cm−1 for the primary amine groups. The oxime derivatives of calix-crown (5a and 5b) bearing butyl amine on the lower rim were synthesized by mixing compound 4a and 4b amphi-monochloro glyoxime in the presence of KOH in MeOH-THF then recrystallization from MeOH-CHCl3 in 49%, 52% respectively. The IR spectra of compound 5a and 5b shows a C=N-bands at 1650 cm−1, 1656 cm−1, respectively. 1H NMR spectroscopy is a versatile tool for the identification of calix[4]arene conformation [33] . But from the 1H NMR spectrum of the compounds 5a and 5b, it is impossible to discern the conformation of calixarene moieties, because the area of Ar-CH2-Ar protons are covered by the protons of CH=N.
Extraction Studies
In our previous work, we stated that oxacrown ethers are regarded as hard ionophores, on the other hand, thiacrown ethers soft ionophores [33] . Therefore, calix-oxacrowns are used alkali metal cations, on the other hand, calix-thiacrown used transition metal cations. The compounds 3a-5a were employed for transferring alkali metal cations, such as Na+, K+ and Cs+, whereas the compounds 3b-4b for transition metal cations such as Hg2+, Cu2+, Cd2+ , Co2+ and Ni2+. The results are summarized by graphic explanation in Figure 1 and Figure 2. These data were obtained by using dichloromethane solutions of the ligands to extract metal picrates from aqueous solution. The equilibrium concentration of picrate in aqueous phase was then determined spectrophotometrically.
From the extraction data shown in Figure 1, it was observed that calix[4]arene-oxacrown-4 2a shows selectivity toward Na+ metal ions from alkali
Figure 1. Extraction percentage of the metal picrates by 2a-5a. Aqueous phase [metal nitrate] = 1 × 10−2 M; [picric acid] = 2.5 × 10−5 M; organic phase, dichloromethane [ligand] = 1 × 10−3 M at 25˚C for 1 h.
Figure 2. Extraction percentage of the metal picrates by 2b-5b. Aqueous phase [metal nitrate] = 1 × 10−2 M; [picric acid] = 2.5 × 10−5 M; organic phase, dichloromethane [ligand] = 1 × 10−3 M at 25˚C for 1 h.
metals. It has been thought that this high selectivity of 2a for Na+ against K+ is due to the appropriate size of 2a, which have a cavity size adjusted to that between Li+ and Na+. It is in agreement with our previously reported study [33] [36] and by literature [37] . Nevertheless dibutyronitrile derivative of this compound (3a) was selective for Cs+ ion as well as Na+ ion. In this case, π-bounds which are present in the structure of nitrile groups play an important role. Because π-bounds are regarded as soft donors according to the HSAB principle [38] , and they favor complexation with the more polarizable metal ions which have larger atomic radius such a Cs+ ion. As far as its oxime derivative (5a), it extracted all of the three metals to the organic phase from the aqueous phase without any selectivity. It is explained by the cation-π interactions, since the metal ion was bound between the two opposite C=O or C=N ligating moieties of these compounds. Similar results were obtained by Vicens and co-workers [39] who had studied various schiff base derivatives of calix[4]arene.
It is seen from the extractions results that the composition of p-tert-butylcalix [4] (tia) crown-4 (2b) carries more Hg 2+ cation than the others (Figure 2). This is an expected result because it carries a soft atom such as sulfur, so it is normal to be interested in a soft metal such as Hg2+. It is not correct by these compounds to only explain the transport of transition metals by the radius of the cation. This is because although the radius of the Hg2+ cation is very close to Cd2+, the extraction rates are very different (Hg2+ 75.4%, Cd2+ < 1.0 %). This is in agreement with the literature [40] . It is also seen that the selectivity of Hg2+ for the nitrile derivative 3b of this compound is still high, while the compound 4b extracts all of the metals used more or less without any selectivity. The oxime derivative, however, extracts all the metals used from the aqueous phase to the organic phase in a good way.
Acknowledgements
We thank the Scientific and Technical Research Council of Turkey (TUBITAK-Number TBAG 105T433) for financial support of this work.
Cite this paper
Akkuş, G.U. and Aslan, S. (2017) Synthesis and Extraction Studies of Calix[4]-Crown-4 Oxime Derivatives. International Journal of Organic Chemistry, 7, 301-311. https://doi.org/10.4236/ijoc.2017.74024
References
- 1. Memon, S., Ali Bhatti, A. and Memon, N. (2013) New Calixarene Appended Amberlite XAD-4 Resin with Versatile Perchlorate Removal Efficiency. Journal of Chemical Engineering Data, 58, 2819-2827. https://doi.org/10.1021/je400554q
- 2. Bhatti, A.A., Memon, S. and Memon, N. (2014) Dichromate Extraction by Calixarene Appended Amberlite XAD-4 Resin. Separation Science and Technology, 49, 664-672. https://doi.org/10.1080/01496395.2013.862722
- 3. Kaya, A., Alpoguz, H.K. and Yilmaz, A. (2013) Application of Cr (VI) Transport through the Polymer Inclusion Membrane with a New Synthesized Calixarene Derivative. Industrial Engineering Chemistry Research, 52, 5428-5436.https://doi.org/10.1021/ie303257w
- 4. Gao, J. Sun, S.P., Zhu, W.P. and Chung, T.S. (2014) Polyethyleneimine (PEI) Cross-Linked P84 Nanofiltration (NF) Hollow Fiber Membranes for Pb2+ Removal. Journal of Membrane Science, 452, 300-310. https://doi.org/10.1016/j.memsci.2013.10.036
- 5. Tabakci, M., Erdemir, S. and Yilmaz, M. (2007) Removal of Dichromate Anions with Nanofiltration-Complexation by Using Amino Calixarene Derivative. Separation Science and Technology, 42, 3321-3331. https://doi.org/10.1080/01496390701626826
- 6. Yilmaz, A., Tabakci, B., Akceylan, E. and Yilmaz, M. (2007) Synthesis and Dichromate Anion Extraction Ability of p-tert-butylcalixarene Diamide Derivatives with Different Binding Sites. Tetrahedron, 63, 5000-5005.https://doi.org/10.1016/j.tet.2007.03.138
- 7. Dung, N.T. and Ludwig, R. (1999) Solvent Extraction of Heavy Metals with Macrocyclic Ligands Based on Calixarenes. New Journal of Chemistry, 23, 603-607.https://doi.org/10.1039/a809771b
- 8. Casnati, A., Massera, C., Pelizzi, N., Stibor, I., Pinkassik, E., Ugozzoli, F. and Ungaro, R. (2002) A Novel Self-Assembled Supramolecular Architecture Involving Cation, Anion and a Calixarene Heteroditopic Receptor. Tetrahedron Letters, 43, 7311-7314. https://doi.org/10.1016/S0040-4039(02)01748-3
- 9. Akceylan, E., Yilmaz, A. and Yilmaz, M. (2013) Synthesis and Properties of Calixarene Polymers Containing Amide Groups: Exploration of Their Extraction Properties towards Dichromate and Nitrite Anions. Macromolecular Research, 21, 1091-1096. https://doi.org/10.1007/s13233-013-1152-0
- 10. Memon, S., Tabakci, M., Roundhill, D.M. and Yilmaz, M. (2005) A Useful Approach toward the Synthesis and Metal Extractions with Polymer Appended Thioalkyl Calixarenes. Polymer, 46, 1553-1560. https://doi.org/10.1016/j.polymer.2004.12.019
- 11. Wanda, S. and Girek, T. (2010) Calixarene Complexes with Metal Ions. Journal of Inclusion Phenomena and Macrocyclic Chemistry, 66, 15-41. https://doi.org/10.1007/s10847-009-9678-7
- 12. Asfari, Z., Wenger, S. and Vicens, J. (1994) Calixcrowns and Related Molecules. Journal of Inclusion Phenomena and Molecular Recognition in Chemistry, 19, 137-148. https://doi.org/10.1007/BF00708979
- 13. Casnati, A., Pochini, A., Ungaro, R., Ugozzoli, F., Arnaud, F., Fanni, S. and Reinhoudt, D.N. (1995) Synthesis, Complexation, and Membrane Transport Studies of 1, 3-alternate calixarene-crown-6 Conformers: A New Class of Cesium Selective Ionophores. Journal of the American Chemical Society, 117, 2767-2777. https://doi.org/10.1021/ja00115a012
- 14. Lamare, V., Bressot, C., Dozol, J.F., Vicens, J., Asfari, Z., Ungaro, R. and Casnati, A. (1997) Selective Extraction of Cesium at Tracer Level Concentration from a Sodium Nitrate Solution with Calix-Crowns. Molecular Modeling Study of the Cs+/Na+ Selectivity. Separation Science and Technology, 32, 175-191. https://doi.org/10.1080/01496399708003193
- 15. Ghidini, E., Ugozzoli, F., Ungaro, R., Harkema, S., Abu El-Fadl, A. and Reinhoudt, D.N. (1990) Complexation of Alkali Metal Cations by Conformationally Rigid, Stereoisomeric calixarene Crown Ethers: A Quantitative Evaluation of Preorganization. Journal of the American Chemical Society, 112, 6979-6985. https://doi.org/10.1021/ja00175a035
- 16. Mokhtari, B. and Pourabdollah, K. (2011) Effect of Crown Size and Upper Moieties in Nano-Baskets of Diacid calixarene-1, 2-crowns-3, 4, 5, 6 on the Extraction of s-Block Metals. Journal of Coordination Chemistry, 64, 3081-3091. https://doi.org/10.1080/00958972.2011.613462
- 17. Vicens, J. and Bohmer, V. (2012) Calixarenes: A Versatile Class of Macrocyclic Compounds. Vol. 3, Springer Science Business Media.
- 18. Luo, J., Zheng, Q.Y., Chen, C.F. and Huang, Z.T. (2005) Synthesis and Optical Resolution of a Series of Inherently Chiral calixcrowns with Cone and Partial Cone Conformations. Chemistry—A European Journal, 11, 5917-5928. https://doi.org/10.1002/chem.200500272
- 19. Xu, C., Yuan, L., Shen, X. and Zhai, M. (2010) Efficient Removal of Caesium Ions from Aqueous Solution Using a Calix Crown Ether in Ionic Liquids: Mechanism and Radiation Effect. Dalton Transactions, 39, 3897-3902.
- 20. Akkus, G.U., Memon, S., Sezgin, M. and Yilmaz, M. (2009) Synthesis of calix (aza) Crown and Its Oligomeric Analogue for the Extraction of Selected Metal Cations and Dichromate Anions. CLEAN—Soil, Air, Water, 37, 109-114. https://doi.org/10.1002/clen.200800120
- 21. Uysal Akku, G., Al, E. and Korcan, S.E. (2015) Selective Extraction of Toxic Heavy Metals and Biological Activity Studies using Pyrimidylthioamide Functionalised Calixarene. Supramolecular Chemistry, 27, 522-526. https://doi.org/10.1080/10610278.2015.1020944
- 22. Guillon, J., Léger, J.M., Sonnet, P., Jarry, C. and Robba, M. (2000) Synthesis of Cone, Partial-Cone, and 1, 3-alternate 25, 27-bis [1-(2-ethyl) hexyl]-and 25, 27-bis [1-(2-tert-butoxy) ethyl] calixarene-crown-6 Conformers as Potential Selective Cesium Extractants. The Journal of Organic Chemistry, 65, 8283-8289. https://doi.org/10.1021/jo001023z
- 23. Li, H., Tian, D., Xiong, D. and Gao, Z. (2007) Synthesis of calixcrown-4 Oligomers Containing Hard and Soft Ion Binding Sites. Journal of Applied Polymer Science, 104, 3201-3205. https://doi.org/10.1002/app.26115
- 24. Levitskaia, T.G., Lamb, J.D., Fox, K.L. and Moyer, B.A. (2002) Selective Carrier-Mediated Cesium Transport through Polymer Inclusion Membranes by calixarene-crown-6 Carriers from Complex Aqueous Mixtures. Radiochimica Acta, 90, 43-52. https://doi.org/10.1524/ract.2002.90.1_2002.43
- 25. Luo, H., Dai, S., Bonnesen, P.V., Buchanan, A.C., Holbrey, J.D., Bridges, N.J. and Rogers, R.D. (2004) Extraction of Cesium Ions from Aqueous Solutions using Calixarene-bis (tert-octylbenzo-crown-6) in Ionic Liquids. Analytical Chemistry, 76, 3078-3083. https://doi.org/10.1021/ac049949k
- 26. Moyer, B.A., Birdwell Jr, J.F., Bonnesen, P.V. and Delmau, L.H. (2005) Use of Macrocycles in Nuclear-Waste Cleanup: A Realworld Application of a Calixcrown in Cesium Separation Technology. In: Macrocyclic Chemistry, Springer Netherlands, 383-405. https://doi.org/10.1007/1-4020-3687-6_24
- 27. Roundhill, D.M., Solangi, I.B., Memon, S., Bhanger, M.I. and Yilmaz, M. (2009) The Liquid-Liquid Extraction of Toxic Metals (Cd, Hg and Pb) by Calixarenes. Pakistan Journal of Analytical and Environmental Chemistry, 10, 1-13.
- 28. Tabakci, M., Memon, S., Yilmaz, M. and Roundhill, D.M. (2004) Oligomeric calixarene-thiacrown Ether for Toxic Heavy Metals. Journal of Polymer Science Part A: Polymer Chemistry, 42, 186-193. https://doi.org/10.1002/pola.11004
- 29. Mokhtari, B., Pourabdollah, K. and Dallali, N. (2011) A Review of Calixarene Applications in Nuclear Industries. Journal of Radioanalytical and Nuclear Chemistry, 287, 921-934. https://doi.org/10.1007/s10967-010-0881-1
- 30. Deligoz, H. (1999) Synthesis of New Vic-Dioxime Derivatives of p-(tert-Butyl) Calixarene and Some Metal Complexes. Organic Preparations and Procedures International, 31, 173-179. https://doi.org/10.1080/00304949909355707
- 31. Deligoz, H. and Yilmaz, M. (1996) Synthesis and Ion Binding Properties of a p-Keto-oxime and a vic-Dioxime Derivative of Calixarene. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry, 26, 943-953. https://doi.org/10.1080/00945719608004345
- 32. Gutsche, C.D. (1983) Calixarenes. Accounts of Chemical Research, 16, 161-170. https://doi.org/10.1021/ar00089a003
- 33. Akkus, G.U., Memon, S. and Yilmaz, M. (2002) Synthesis of Oligomeric Calixarene-crowns as Novel Ionophores for Alkali and Transition Metals. Polycyclic Aromatic Compounds, 22, 1075-1086. https://doi.org/10.1080/10406630214288
- 34. Pedersen, C.J. (1967) Cyclic Polyethers and Their Complexes with Metal Salts. Journal of the American Chemical Society, 89, 7017-7036. https://doi.org/10.1021/ja01002a035
- 35. Inoue, K., Hirakawa, H., Ishikawa, Y., Yamaguchi, T., Nagata, J., Ohto, K. and Yoshizuka, K. (1996). Adsorption of Metal Ions on Gallium (III)-Templated Oxine Type of Chemically Modified Chitosan. Separation Science and Technology, 31, 2273-2285. https://doi.org/10.1080/01496399608001046
- 36. Memon, S., Uysal, G. and Yilmaz, M. (2001) Syntheses and Binding Properties of Polymeric calixcrown-4. Reactive and Functional Polymers, 47, 165-174.
- 37. Shinkai, S. (1991) Functionalized Calixarenes: New Applications as Catalysts, Ligands, and Host Molecules. Springer, New York, 173-198.
- 38. Pearson, R.G. (1963). Hard and Soft Acids and Bases. Journal of the American Chemical Society, 85, 3533-3539. https://doi.org/10.1021/ja00905a001
- 39. Seangprasertkij, R., Asfari, Z., Arnaud, F. and Vicens, J. (1994) Schiff Base p-tert-butylcalixarenes. Synthesis and Metal Ion Complexation. The Journal of Organic Chemistry, 59, 1741-1744. https://doi.org/10.1021/jo00086a024
- 40. Yordanov, A.T., Mague, J.T. and Roundhill, D.M. (1995) Synthesis of Heavy Metal Ion Selective calixarenes Having Sulfur Containing Lower-Rim Functionalities. Inorganic Chemistry, 34, 5084-5087. https://doi.org/10.1021/ic00124a027



