Synthesis and Antibacterial Studies of Metal Complexes of Cu(II), Ni(II) and Co(II) with Tetradentate Ligand ()
1. Introduction
Metal complexes of Schiff base ligands possess a variety of applications in the biological, analytical, clinical, and industrial areas [1] . In recent times, transition metal complexes of Schiff base ligands have gained considerable attention, not only due to their spectroscopic properties and applications [2] but also due to their remarkable antifungal, antibacterial and antitumor activities [3] . Schiff bases have a vital position in metal coordination chemistry even almost a century since their discovery. Due to their simplicity in preparation, diverse properties, medicinal, biochemical and industrial applications, the keen interest in the study of these compounds arose in the recent years. A number of metal coordination complexes of Schiff bases have been suggested as antibacterial, antifungal, cytotoxic, anti-inflammatory and cytostatic agents [4] [5] [6] [7] . In order to widen the scope of investigations on the coordination behavior of various donor ligands including Schiff base towards organo metallics, we carried out the investigations and established their bioactivities [8] [9] [10] [11] . This paper describes the synthesis of Cu(II), Ni(II) and Co(II) transition metal complexes with the new ligand namely 3,3’-(cyclohexane-1,2-diylbis(azanylylidene))bis(indolin-2- one) and its characterization by NMR, FT-IR, UV-VIS and elemental analysis, as well as the antibacterial studies.
2. Experimental
Isatine, 1,2-diaminocyclohexane, cesium iodide, Metals salts [CoCl2・6H2O, CuCl2・2H2O and NiCl2・6H2O], ethanol, DMF, DMSO, benzene, Ether, acetone, Ether ,dichloromethane, chloroform and methanol are from supplied by BDH or Sigma-Aldrich and utilized without purifications. Infra-red spectra for ligand and complexes were done by utilizing a Shimadzu FT-IR-8300 Spectrophotometer. 1H-NMR spectra for ligand and complexes were done by utilizing BrukerDPX-300 MHz Spectrophotometer and the internal standard was TMS. Ultraviolet spectra for ligand and complexes were obtained by utilizing of Shimadzu Ultraviolet Visible-160A spectrophotometer. Magnetic susceptibilities that obtained for the complexes were done at room temperature by utilizing Magnetic Susceptibility Balance-MSBMKI. CHN-analysis was done for the ligand and complexes utilizing elemental analyzer-5500 Carlo Erba.
2.1. Synthesis
2.1.1. Synthesis of the Ligand 3,3’-(Cyclohexane-1,2-Diylbis (Azanylylidene))Bis(Indolin-2-One)
Schiff base was prepared by condensation of isatine (0.010 mol) with 1,2-di- aminocyclohexane (0.005 mol) in ethanol (20 ml) with 3 drops of glacial acetic acid and the mixture was stirred at ambient temperature for 6 hours. The progress of reaction was monitored by TLC. On completion of reaction the product was separated as crystals which were filtered, dried, and washed with cold water.
2.1.2. Synthesis of the Complexes
[Co(Ligand)Cl2], [Ni(Ligand)] and [Cu(Ligand)Cl2] complexes were prepared by the addition of 1mmol of metal chlorides Cu(II), Ni(II), or Co(II), which were dissolved in about 15 ml of water, into a hot ethanol solution of 1mmol of the ligand. The mixture was then refluxed for 2 h. The precipitated solids were filtered off from the ice-cooled reaction mixture. The solids were washed with ether and filtered.
2.2. Antibacterial Activities
Antibacterial activity of the ligand and complexes were determined by the agar well diffusion method [12] . E. coli, P. aeruginosa and S. aureus. Strains of three bacterial species which included Gram-positive bacteria, namely Staphylococcus aureus and Gram-negative bacteria namely Pseudomonas aeruginosa and Escherichia coli were investigated. Ciprofloxacin was used as the standard antibacterial agents. The bacteria isolates were subcultured on nutrient agar plates and incubated at 37˚C for 24 h. A loop full of bacteria cells from the nutrient agar plates was incubated into a nutrient broth (50 ml) at 37˚C for 18 h with vigorous shaking. Using a sterile glass spreader, 18 h bacterial cultures (100 µl) were used to spread a bacterial lawn on nutrient agar [13] . The bacterial strains were grown at 37˚C overnight and maintained on nutrient agar. A stock solution of the compounds were prepared in DMF at 50˚C to give a final concentrations; after pouring into plates and allow the agar to set, plates were inoculated with standardized inocula of the test bacteria, and further incubated at 37˚C for 24 h under aseptic conditions.
3. Results and Discussions
The ligand 3,3’-(cyclohexane-1,2-diylbis (azanylylidene))bis(indolin-2-one) was prepared by reaction of amounts isatine and 1,2-diaminocyclohexane in ethanol (Scheme 1). It was shine crystals, soluble in dimethylformamide (DMF), dimethylsulfoxide (DMSO), hot methanol, hot ethanol, acetone and dichloromethane, but it is insoluble in water, hexane and diethylether. The ligand was characterized by means of elemental analysis, infrared spectrum, Uv-Vis and NMR spectroscopes. Elemental analysis data for the synthesized ligand, Table 1, is in consistent with the suggested stoichiometry.
3.1. 1H-NMR Spectroscopy
The NMR show doublet-doublet for methylene groups (CH2; 2H) at 1.82 ppm and multiple at 2.35 - 2.40 for (CH2; 2H), 4.27 ppm, doublet-doublet for protons CH. While the protons at 7.31 - 7.67 ppm were for aromatic ring while amino group located at 8.02 ppm.
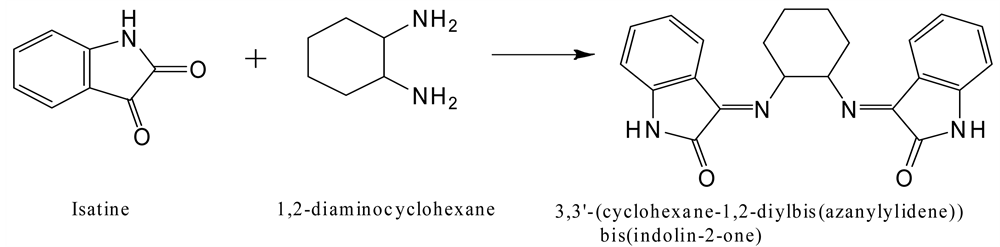
Scheme 1. Synthetic procedure used for preparation Schiff base derivative.
![]()
Table 1. Analytical data of ligand and its complexes.
3.2. FT-IR Spectroscopy
The infrared spectrum of the ligand, showed four characteristic bands at 3316 cm−1, 3072 cm−1, 1661 cm−1 and 1544 cm−1, Table 2, which are attributed to amine band (N-H), aromatic band (C-H aromatic), carbonyl band (C=O) and azomethane band (C=N) respectively. The presence of these four bands in the FT-IR spectrum of this ligand indicates the reaction of isatine and 1,2-diamino- cyclohexane and formation of azomethane group in the ligand. The primary (NH2) group of the reactant isatine has two IR bands and upon reaction with 1,2-diaminocyclohexane, these two bands are disappeared and a band at 3316 cm−1, Table 2, is appeared which belongs to υ (N-H) of the secondary amine (N-H) of the ligand. This is an indication of the reaction of the isatine with 1,2-diaminocyclohexane and formation of the ligand.
Treatment of the ligand with metal chloride of (Co(II), Ni(II) and Cu(II)) in a solvent gave complexes of the types [M(Ligand)Cl2], where M = Co(II) and Cu(II), [M(Ligand)], where M = Ni(II). The prepared metal complexes are characterized as follow: Elemental analysis data for the synthesized ligand and its metal complexes are shown in Table 1. The metal complexes are consistent with the suggested stoichiometries.
Molar conductivities of all synthesized complexes are measured for (1 × 10−3 M) solution in DMSO at room temperature, Table 1 and indicates that they are nonelectrolyte and consistent with the proposed formula for them. The infrared spectra of the prepared ligand and its metal complexes are measured in the range (200 - 4000) cm−1 using cesium iodide (CsI) disc. The characteristic band frequencies are arranged in Table 2.
The infrared spectral data were discussed according to the functional groups present in the prepared complexes as follow: IR data given in Table 2 show broadening of ?N=C and carbonyl peaks, which suggest coordination through these sites. While the peak of −NH remains unaltered, and the peak of non- coordinated carbonyl diminishes due to tautomerism, it can be proposed that the carbonyl peak diminishes due to hydrogen bond formation with free noncoordinated secondary amine group. Therefore, the proposed structure of the coordination complexes produced is given as shown in Figure 1.
3.3. Conductance
Conductance of the complexes are given in Table 1, conductance data show that the metal complexes are non electrolyte indicating the chloride are located inside
![]()
Table 2. IR spectra for ABH and its complexes (cm−1) of selected region.
![]()
Figure 1. Proposed structure of metal(II) complexes.
the coordination sphere and are directly involved in coordination with the metal center except Ni(II).
3.4. Magnetic Susceptibility
The magnetic susceptibility of synthesized complexes is shown in Table 1. The magnetic susceptibility of Co(II) complex is 4.3 B.M [14] , which denotes to an octahedral geometry. The magnetic susceptibility of Ni(II) complex, 3, is 0.06 B.M, which indicates a square-planar geometry [15] . The magnetic susceptibility of Cu(II) complex, is 3.67 B.M, which suggests an octahedral geometry arrangement around Cu(II) [16] .
3.4.1. Co-Complex
The electronic spectrum of Co-complex shows two bands in Uv-Vis. region at 299 nm and 249 nm are assigned to charge transfer transitions, while the band at 496 nm is assigned to transition 4T1g(F) → 4T1g(P) of Co(II) in an octahedral arrangement (Figure 2).
3.4.2. Ni-Complex
The electronic spectrum of the Ni-complex, show bands at 439 nm and 573 nm which assigned to 3T1(F) → 3T1(P) and 3T1(F) → 3T2(F) transition probably indicating square-planar geometry (Figure 3).
3.4.3. Cu-Complex
The electronic spectrum of the Cu-complex shows bands at 544 and 610 nm which indicating an octahedral arrangement (Figure 4).
3.5. Antibacterial Activities
The treatment of infectious diseases still remains an important and challenging problem because of a combination of factors including emerging infectious diseases and the increasing number of multi-drug resistant microbial pathogens. In spite of a large number of antibiotics and chemotherapeutics available for medical use, at the same time the emergence of old and new antibiotic resistance created in the last decades revealed a substantial medical need for new classes of antimicrobial agents. There is a real perceived need for the discovery of new compounds endowed with antimicrobial activity, possibly acting through
![]()
Figure 2. The geometrical structure for Co-Complex.
![]()
Figure 3. The geometrical structure for Ni-Complex.
![]()
Figure 4. The geometrical structure for Cu-Complex.
mechanism of action, which is distinct from those of well-known classes of antimicrobial agents to which many clinically relevant pathogens are now resistant [17] [18] [19] . The field of bioinorganic chemistry, which deals with the study of role of metal complexes in biological systems, has opened a new horizon for scientific research in coordination compounds. A large number of compounds are important from the biological point of view [20] [21] . The results of in vitro antibacterial activity of the ligand and its Co(II), Ni(II) and Cu(II) complexes are presented in Figure 5. Amoxicillin was used as positive standards and DMSO was used as negative control for these antibacterial activities. In general, metal (II) complexes have been shown to be more effective than the free ligands and the same was observed in this study, i.e., that the complexes are more active than the parent ligand. The Cu(II) and Co-complexes were found to show bactericidal activity against selected types of bacteria. A fairly moderate effectiveness is exhibited by Ni(II) complex towards E. coli. The ligand show less activity towards E. coli and P. aeruginosa respectively, but had no activity against the organisms E. coli within the range of concentrations considered. The variation in the activity
![]()
Figure 5. The Antibacterial activities of the ligand and metal complexes in mm.
of different complexes against different organisms depends either on the impermeability of cells of the microbes or differences in the ribosomes of microbial cells [22] . The higher antibacterial activity of M-complexes than the free Schiff base ligands can be explained by chelation of the ligand with metal ions [23] as metal chelates display both polar and nonpolar properties; this makes them suitable for permeation into cells and tissues. The polarity of the metal ion will be reduced to a greater extent due to the overlap of the ligand orbital upon chelation, and partial sharing of the positive charge of the metal ion with donor groups. Chelation increases the delocalization of π-electrons over the entire chelate ring and enhances the penetration of the complexes into lipid membranes [23] [24] . It also increases the hydrophilic and lipophilic nature of the central metalions, probably leading to lipo-solubility and permeability through the lipid layer of cell membranes. Further, lipophilicity, which controls the rate of entry of molecules into the cell, is modified by coordination, so the metal complex can become more active than the free ligand [25] .
4. Conclusion
The synthesized complexes of 3,3’-(cyclohexane-1,2-diylbis(azanylylidene))bis- (indolin-2-one) ligand show octahedral geometries for Co(II) and Cu(II), but square planer for Ni(II). Magnetic moment studies and conductance studies prove the assigned geometries. Ligand and the synthesized complexes were tested as antibacterial agents. In general, the antimicrobial activity of metal complexes is depended more on the metal center itself than on the geometry around the metal ion.
Acknowledgements
The author thanks the Iraqi Government, Ministry of Higher Education, Basrah University and Polymer Research Centre for the financial support provided for this work.