Assessment of Groundwater Quality in the Dogger Aquifer of Poitiers, Poitou-Charentes Region, France ()
1. Introduction
Groundwater is an essential source of water supply in many parts in the world due to its large volumes and its low vulnerability to pollution when compared to surface waters [1] . Nowadays, groundwater resources play an important role in meeting demands on water supply because of scanty surface-water sources or their unsuitability. Due to population growth and increase of economic activities (industries, agriculture), groundwater resources are more and more under stresses. They are over-exploited leading to critical levels. Pollution of groundwater is also a major issue because these resources are inherently susceptible to contamination from land use and anthropogenic and other activities impacts. In agricultural areas, the use of fertilizers is a main source of groundwater pollution. Once pollution enters the subsurface environment, it may remain concealed for many years and render groundwater supplies unsuitable for consumption and other uses. Preventing groundwater pollution is necessary for effective groundwater resource management. An initial step toward sustainable groundwater resource management involves understanding groundwater quality and processes of mineralization.
This paper is focused on the Dogger aquifer of Poitiers, in the Poitou-Charentes Region, Center West of France (Figure 1). The Region is made up of 4 Departments (Vienne, Charente, Charente Maritime, Deux- Sèvres). The Dogger aquifer is of vital importance for the socio-economic development of this region, specifically for the Vienne Department, supplying the needs of almost the entire domestic and irrigation demands. Total water volumes collected in 2010 amounted to 81 million m3, and are mainly used for drinking water and agriculture (Table 1). 66 million m3 are taken from groundwater, which is more than 80% of the total volume. Agriculture is the dominant economic activity in the region and uses 48 million m3, which represents 60% of the total volume. Groundwater accounts for 81% of this volume. These statistics place great emphasis on the vital importance of groundwater for this region. This study attempts to evaluate the hydrogeochemical processes controlling the groundwater composition in this unconfined aquifer.
![]()
Figure 1. Location of the study area in the Vienne department, Poitou-Charentes region, center west France.
2. Study Site: Geology and Hydrogeology
The study area is located between two main rivers, Clain and Vienne, and receives an average annual rainfall of 750 mm. The surface of the study area is about 500 km2. The study area is at the crossroads of four major geological units: the sedimentary formations of the Paris Basin in the north-east and the Aquitaine Basin in the south-west and the schist and granite massifs of the Armorican Massif to the north-west, and the Central Massif in the south east. The threshold of Poitou is at the interface of these units (Figure 2). From a geological point of view, in the study area, the Mesozoic series covers the granite basement previously subjected to severe erosion. Above this basement, which outcrops locally in the valleys (Ligugé Champagné St Hilaire...), lies the Lias series which consist of various facies (clay, sand, limestone and dolomites). The Infra-Toarcian contains a confined aquifer with a relatively thin (tens of meters) but karstic and fractured reservoir. It is confined under marly formations (Figure 2).
Above the Lias, lies the Dogger limestones in which karst is well developed. This karst massif is in the form of plateaus. The limestones are overlain by sandy clay soil, alteration products combined with fluvial and aeolian deposits. These formations, which coverall most all the Dogger, store rain water but generally have poor permeability. They slowly supply with water the underlying karst aquifers evidenced by the many karstic features (sinkholes, closed depressions...) that can be observed. To the north, more recent formations outcrop: the Upper Jurassic limestones (Malm), and Cretaceous clays and limestones.
The confined Lias aquifer is unaffected by surface pollution. However, it has undesirable natural elements (fluorine, arsenic) which often exceed the standards for drinking water concentration. The limestone aquifer of the Middle Jurassic (Dogger) is the main water resource whether for irrigation or drinking water. This particularly vulnerable karstic and unconfined aquifer of ten has a poor quality (contamination by nitrates and pesticides from agriculture). The thickness of this aquifer is important to the north and south of the basin, but is strongly reduced in the central part (bulging of Poitou threshold) and even disappears completely at the Champagné Saint Hilaire and Ligugehorsts (Figure 2). Alluvial aquifers and that of the Cretaceous (Cenomanian), downstream of Poitiers, are unproductive.
A piezometric campaign of the Dogger aquifer has been conducted in November 2012. Around one hundred wells and boreholes have been inventoried and helped establish the piezometric map of the groundwater (Figure 3). The piezometric map shows that the groundwater is drained by the Clainriverto the West and the Vienne river to the East. A groundwater dividing line separates the basins of Clain and Vienne. However underground basins do not generally correspond to surface topographic watersheds. Groundwater depth is close to the soil surface in the valleys and ranges between 20 m to 40 m on the plateau between the rivers Clain and Vienne. Groundwater level fluctuates by a few meters on the plateau between low water period (August-September) and high water period (March-April).
3. Methodology
During the campaign of November 2012, 66 wells were sampled and analyzed to understand the chemical variations of groundwater (Figure 4). Samples collected were filtered using 0.45 lm pore size membrane and stored in polyethylene bottles which are initially washed with 10% of HNO3 and rinsed thoroughly with distilled water.
![]()
Figure 2. Geological SW-NE cross-section. Source BRGM, modified.
![]()
Figure 3. Piezometric map of the Dogger groundwater (November 2012).
![]()
Figure 4. Location of the wells sampled for physico-chemical analyses.
Physical parameters like pH, and electrical conductivity, were measured in the field with a multi-parameter set of WTW multi 350i brand. Major cations (Na+, K+, Mg2+, Ca2+) were estimated by atomic absorption. 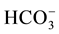 was estimated by titrimetric method.
was estimated by titrimetric method.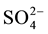 , Cl− and
, Cl− and 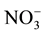 were estimated by chromatography. The analytical precision for the measurement was determined by calculating the ionic balance error, which is generally found to be within ±5%. Graphical methods (Piper diagram, binary diagrams) and statistical methods such as the correlation matrix and the principal components analysis were used to interpret the data.
were estimated by chromatography. The analytical precision for the measurement was determined by calculating the ionic balance error, which is generally found to be within ±5%. Graphical methods (Piper diagram, binary diagrams) and statistical methods such as the correlation matrix and the principal components analysis were used to interpret the data.
4. Results and Discussions
4.1. Descriptive Statistics
Descriptive statistics (average, minimum, maximum, standard deviation) of all the analyzed parameters are reported in Table 2. The groundwater is moderately mineralized (average CE = 694 µS/cm). The standard deviation for all variables is lower than the average value. This indicates that the chemical properties of the Dogger groundwater are not widely dispersed. The coefficient of variation of Mg2+ is the highest (CV = 92.3%), followed by 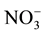 (CV = 83.3%). Cl−, K+ and Na+ have a CV > 60%. The dominant anion is bicarbonate. The order of importance of the anions is the following:
(CV = 83.3%). Cl−, K+ and Na+ have a CV > 60%. The dominant anion is bicarbonate. The order of importance of the anions is the following: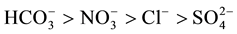 . The average concentrations of
. The average concentrations of ![]() is 48 mg/L, close to the standard value for drinking water which is 50 mg/L. The dominant cation is calcium Ca2+ concentrations are greater than those of Mg2+, Na+ and K+. The order of importance of the cations is
is 48 mg/L, close to the standard value for drinking water which is 50 mg/L. The dominant cation is calcium Ca2+ concentrations are greater than those of Mg2+, Na+ and K+. The order of importance of the cations is ![]() . The average concentration of Ca2+ is 98 mg/L, much higher than the average concentration of other cations (Mg2+: 13 mg/L; Na+: 19 mg/L; K+: 30 mg/L).
. The average concentration of Ca2+ is 98 mg/L, much higher than the average concentration of other cations (Mg2+: 13 mg/L; Na+: 19 mg/L; K+: 30 mg/L).
4.2. Diagram of Piper
Results of the chemical analyses (major ions), plotted on the Piper diagram (Figure 5), clearly reveal a certain homogeneity of the Dogger groundwater hydrochemical composition. All samples display two major ions, bicarbonate and calcium. This shows that the calcium bicarbonate facies is the dominant one in this aquifer, except for two particular wells, 51 and 28. The concentrations of chemical elements in these two samples are abnormal. Well #28 has a sodium bicarbonate facies. This well is located in Poitiers city, in Callovian limestones. The sodium concentration in this well is very high and equal to 134 mg/L. Well #51 has a chloride-sulfate and calcium- magnesium facies. This well is located in a part of the aquifer covered by tertiary formations. The groundwater presents several elements in excess concentrations. The chloride content (278 mg/L) is much higher than the maximum value observed elsewhere (77 mg/L) on the remaining samples. Also the sulphate content reaches 55 mg/L, exceeding that of the other samples. Similarly, the potassium reaches 271.3 mg/L. The presence of this facies can be linked to a very particular pollution from irrigation water.
It is assumed that chloride is not affected by chemical processes such as dissolution, precipitation, and especially hydro-morphic substitutions. So in a remote aquifer from marine influences, the origin of the chlorides can be mainly anthropogenic. Therefore these two wells were removed from the data for subsequent interpretation.
4.3. Saturation Index
The saturation index approach allows predicting the reactive mineralogy of the subsurface from groundwater data without collecting the samples of the solid phase and analyzing the mineralogy. The saturation index SI of a mineral is calculated based on the following equation [2] :
![]()
where IAP is the ion activity product and Ks is the solubility product of the mineral.
![]()
Table 2. Descriptive statistics of the variables.
![]()
Figure 5. Piper diagram of the water samples taken in November 2012.
The PHREEQC package [3] was used to estimate the saturation index. SI indicates if a solution is in equilibrium, under-saturated or super-saturated with regard to a mineral. Equilibrium can be assumed for a range of ?0.5 to 0.5. If the SI value is below −0.5, the solution is under-saturated with regard to the corresponding mineral, if the SI exceeds +0.5 the water is super-saturated with respect to this mineral. Table 3 shows the statistics of the saturation indices for the minerals calcite, gypsum and halite in the collected water samples. Most of the samples were found in equilibrium or slightly saturated with calcite. The SI for calcite ranges between 0.01 to 1.2. The average SI for calcite is 0.72. The SI for gypsum and halite ranges between −2.78 to −1.71 and −9.44 to −7.13 respectively. The average SI is −2.20 for gypsum and −9.44 for halite. This indicates that the groundwater is under-saturated with regards to these minerals. These findings explain why the major ions dissolve in the Dogger groundwater are Ca2+, and![]() .
.
4.4. Correlation Matrix
A correlation matrix was computed considering all the physico-chemical variables (Table 4). This is in perspective to better appreciate associations among the various parameters. The critical value of the correlation coefficient, at 1% level of significance, was calculated using the following transform:
![]()
where Robs is the observed correlation coefficient, n is the sample size, t is a statistics which follows a Student law with n ‒ 2 degrees of freedom. Details can be found in [4] . This yielded a critical correlation coefficient value (Rcritical) of 0.32. Thus values of R less than 0.32 are meaningless. Significant values of R are highlighted in the correlation matrix (Table 4).
The correlation matrix shows that the electrical conductivity is correlated with bicarbonate (R = 0.67), sulphate (R = 0.46), nitrate (R = 0.48), chloride (R = 0.48), calcium (R = 0.50) and potassium (R = 0.61). The groundwater mineralization is mainly related to these elements. Bicarbonate is correlated with calcium (R = 0.45), magnesium (R = 0.38) but also with potassium (R = 0.48). Sulphate displays significant correlations with chloride (R = 0.43) and sodium (R = 0.62). Chloride is well correlated with calcium (R = 0.50) and potassium (R = 0.42), and nitrate with chloride (R = 0.34) and calcium (R = 0.49). Some significant relations are related to the geological environment (![]() , Ca2+, Mg2+). Other relations exist between ions of same sign (
, Ca2+, Mg2+). Other relations exist between ions of same sign (![]() - Cl−) or ions of opposite sign (
- Cl−) or ions of opposite sign (![]() - Na+;
- Na+; ![]() - Ca2+; Cl− - Ca2+; Cl− - K+). These relationships allow assuming a common origin for these elements from agricultural activities disturbing the natural hydrochemistry of the Dogger groundwater.
- Ca2+; Cl− - Ca2+; Cl− - K+). These relationships allow assuming a common origin for these elements from agricultural activities disturbing the natural hydrochemistry of the Dogger groundwater.
![]()
Table 3. Descriptive statistics of the saturation indices.
![]()
Table 4. Correlation matrix between variables.
4.5. Principal Component Analysis
Principal Component Analysis (PCA) is a multivariate statistical method which has been successfully applied to study the hydrogeochemistry of aquifers [5] - [10] . The principle is to reduce the number of original variables by detecting the underlying relations. PCA explains a large amount of the variance of the analytical data by a small number of factors or principal components [6] [11] . The factor extraction is done using a minimum acceptable eigen value which should be greater than 1 [12] [13] .
Table 5 shows the 5 first eigen values and the amount of total variance explained by each of them. The first three eigen values greater than one were kept for the analysis. Table 6 shows the factor loadings for each variable. Factor loading is the measure of the degree of relation between the variables and the factor.
Plots of the variables on the planes associated with factors 1-2 and factors 1-3 are shown on Figure 6. Factor 1 has high loadings for EC, ![]() , Ca2+, Cl−, Na+,
, Ca2+, Cl−, Na+, ![]() ,
, ![]() , and K+, except for Mg2+ and Na+. This factor is related mainly with the mineralization of the samples. The substantial contribution of nitrate in the study area results from the application of agricultural fertilizers in excess of the land capability conditions [14] .
, and K+, except for Mg2+ and Na+. This factor is related mainly with the mineralization of the samples. The substantial contribution of nitrate in the study area results from the application of agricultural fertilizers in excess of the land capability conditions [14] .
Factor 2 has high loadings for![]() , Ca2+ and Na+ and factor 3 for Mg2+ and
, Ca2+ and Na+ and factor 3 for Mg2+ and![]() . Factor 2 highlights some ions association that might be related to the use of fertilizers in the study area (
. Factor 2 highlights some ions association that might be related to the use of fertilizers in the study area (![]() , Ca2+) or to base exchanges processes (Na+). Factor 3 is related to Mg2+ and
, Ca2+) or to base exchanges processes (Na+). Factor 3 is related to Mg2+ and![]() , which may result from the influx of these ions by dissolution from dolomitic rocks.
, which may result from the influx of these ions by dissolution from dolomitic rocks.
4.6. Chloro-Alkaline Index
The chloro-alkaline index (CAI) was used to define the ionic exchanges between water and the aquifer rocks [15] . The expression of the chloro-alkaline index (CAI) is as follows:
![]()
This ratio is positive when the sodium and potassium contents are low, i.e. when groundwater has been slightly in contact with minerals able to release these interchangeable cations easily. These exchanges of ions
![]()
Table 5. Eigen values and explained variance.
![]()
Table 6. Factor loadings for the variables.
![]()
![]()
Figure 6. Principal component analysis. Plot of the variables on the F1-F2 plane and on the F1-F3 plane.
contained in the matrix with those present in the groundwater are very variable and depend, among other things, on the nature of the substrate. This ratio is negative when the sodium and potassium contents are high, i.e. when groundwater has been strongly in contact with minerals able to yield these interchangeable cations easily. In the case of the Dogger aquifer, the base exchange index is negative in 93% of the samples. This indicates that the groundwater exchanged alkaline earths Ca2+ and Mg2+ against the alkalis Na+ and K+ of clays contained in the underground environment, according to the equation below:
![]()
Ca2+ and Mg2+ can exchange Na+ sorbed on the exchangeable sites of the clay minerals, resulting in the increase of Na+ and the decrease of Ca2+ and Mg2+ in groundwater.
The diagram of ![]() versus Na+/Cl−, (Figure 7) shows that the contact of groundwater
versus Na+/Cl−, (Figure 7) shows that the contact of groundwater
with the sodic clayey formations in the aquifer supports the losses of calcium due to the process of base exchanges (Quadrants B and C, Figure 7). Figure 7 clearly depicts that calcium is still in deficiency and sodium is in excess.
4.7. Binary Diagrams
Binary diagrams between chemical elements were drawn to better understand the processes and specify the likely origin of the major element participating to the groundwater mineralization. The plot of Na+ versus Cl− is reported on Figure 8(a). There is no relation between Na+ and Cl− and correlation coefficient is not significant
![]()
Figure 7. Relationship between ![]() and Na+/Cl− ratios.
and Na+/Cl− ratios.
![]() (a) (b)
(a) (b)![]() (c) (d)
(c) (d)
Figure 8. Relationship between Na-Cl (a); Ca-HCO3 (b); Mg-HCO3 (c) and Na-SO4 (d).
(R = 0.29). Most of the data in Figure 8(a) deviate from the expected 1:1 line. The plot shows an excess of Na+ in most of the wells. This excess can be explained by a different origin of this element than the dissolution of halite. The high concentrations of Na+ which come along with the low concentrations of Cl− can be due to the base exchange phenomena because clays of the substratum can release the ions Na+ after having fixed Ca2+. This figure also depicts an enrichment of Cl− in some wells, indicating the effect of another source for this ion than the dissolution of halite. There is a significant relation between Ca2+ and ![]() with a correlation coefficient of R = 0.45. The plot of Ca2+ vs.
with a correlation coefficient of R = 0.45. The plot of Ca2+ vs. ![]() (Figure 8(b)) shows an excess of Ca2+. Almost all samples plot above the 2:1 line. This indicates that calcite may not be the main source of Ca2+. Magnesium and bicarbonate are significantly correlated (R = 0.38). Figure 8(c) indicates a deficiency of Mg2+ relative to
(Figure 8(b)) shows an excess of Ca2+. Almost all samples plot above the 2:1 line. This indicates that calcite may not be the main source of Ca2+. Magnesium and bicarbonate are significantly correlated (R = 0.38). Figure 8(c) indicates a deficiency of Mg2+ relative to ![]() in most of the groundwater samples. This is consistent with the cation exchange between alkaline earths (Ca2+ and/or Mg2+) and alkalis (Na+ and/or K+). The plot of Na+ vs.
in most of the groundwater samples. This is consistent with the cation exchange between alkaline earths (Ca2+ and/or Mg2+) and alkalis (Na+ and/or K+). The plot of Na+ vs. ![]() (Figure 8(d)) exhibits a well-defined relationship (R = 0.62) but also shows an excess of Na+ relative to
(Figure 8(d)) exhibits a well-defined relationship (R = 0.62) but also shows an excess of Na+ relative to![]() . This supports the assumption that the excess of sodium does not result from the dissolution of sodium sulphate minerals (Na2SO4).
. This supports the assumption that the excess of sodium does not result from the dissolution of sodium sulphate minerals (Na2SO4).
4.8. Antropogenic Inputs
Chemical composition of groundwater is generally controlled by inputs through water/rock interaction and also through anthropogenic activities. Variation in TDS in groundwater may be related to land use and also to various contaminants [16] [17] . It is well known that K+, ![]() ,
, ![]() , Na+ and Cl− ions are mostly derived from agricultural fertilizers, animal waste, and municipal sewage. These elements can be used to highlight the influence of human activities on the groundwater chemistry [18] .
, Na+ and Cl− ions are mostly derived from agricultural fertilizers, animal waste, and municipal sewage. These elements can be used to highlight the influence of human activities on the groundwater chemistry [18] .
The Dogger plateau, between the cities of Poitiers and Chauvigny is mainly an agricultural area. Nitrate is commonly and widely applied as a fertiliser. Nitrate can also be derived from other sources like animal excreta and also from nitrification of organic N and ![]() [19] - [22] . Nitrate values in the Dogger aquifer are relatively high. They vary in a wide range, between 7 and 165 mg/L. In a number of wells, nitrate concentrations exceed 50 mg/L, which represents the maximum admissible nitrate concentration in drinking water. The high contents are related to the agricultural practices. This suggests that the application of nitrogen fertilizers leads to increased nitrate leaching. Besides, the
[19] - [22] . Nitrate values in the Dogger aquifer are relatively high. They vary in a wide range, between 7 and 165 mg/L. In a number of wells, nitrate concentrations exceed 50 mg/L, which represents the maximum admissible nitrate concentration in drinking water. The high contents are related to the agricultural practices. This suggests that the application of nitrogen fertilizers leads to increased nitrate leaching. Besides, the ![]() contents in the groundwater correlate positively with the EC values indicating the significant contribution of this element in the mineralization process.
contents in the groundwater correlate positively with the EC values indicating the significant contribution of this element in the mineralization process.
The main cities and villages are equipped with sewage treatment plants. Farms and isolated houses are equipped with individual wastewater treatment systems. It is well known that poorly functioning individual treatment plants, can be responsible for chloride concentration in groundwater
There is a positive and significant correlation between ![]() and Ca2+ (R = 0.49, Figure 9(a)), suggesting a common source. Indeed, the positive relationship between
and Ca2+ (R = 0.49, Figure 9(a)), suggesting a common source. Indeed, the positive relationship between ![]() and Ca2+ suggests that both elements are utilized in the Dogger plateau as Ca(NO3)2 fertilizers. Ca2+ and
and Ca2+ suggests that both elements are utilized in the Dogger plateau as Ca(NO3)2 fertilizers. Ca2+ and ![]() can indeed be associated in some fertilizers. Calcium excess can originate from this source [23] .
can indeed be associated in some fertilizers. Calcium excess can originate from this source [23] .
Positive and significant correlations between ![]() and Cl−,
and Cl−, ![]() and Na+,
and Na+, ![]() and Cl−, Cl− and Ca2+ (Figure 9(b)), Cl− and K+, indicate that these elements derive mainly from anthropogenic activities.
and Cl−, Cl− and Ca2+ (Figure 9(b)), Cl− and K+, indicate that these elements derive mainly from anthropogenic activities.
![]() (a) (b)
(a) (b)
Figure 9. Relationship between ![]() - Ca2+ (a) and Cl− - Ca2+ (b).
- Ca2+ (a) and Cl− - Ca2+ (b).
5. Conclusions
Chemical properties of groundwater in the Dogger aquifer of Poitiers are controlled both by natural geochemical processes and anthropogenic activities. Dissolution of dolomite and calcite determines![]() , Ca2+ and Mg2+ content in the groundwater. Other reactions, such as cation exchange of Na+ for Ca2+ and Mg2+, also contribute to the content of Na+. The main hydrochemical type water is calcium carbonate. The SI of calcite is generally greater than zero in the area, which suggests that the groundwater is saturated with regards to this mineral.
, Ca2+ and Mg2+ content in the groundwater. Other reactions, such as cation exchange of Na+ for Ca2+ and Mg2+, also contribute to the content of Na+. The main hydrochemical type water is calcium carbonate. The SI of calcite is generally greater than zero in the area, which suggests that the groundwater is saturated with regards to this mineral.
The use of nitrate fertilizers in the intensively cultivated area of the study area contributes to the increase of the concentration of![]() . Some elements in excess (Ca2+,
. Some elements in excess (Ca2+, ![]() , K+) may also derive from the application of fertilizers. Poorly treated waste waters can contribute to the input of Cl− in the groundwater.
, K+) may also derive from the application of fertilizers. Poorly treated waste waters can contribute to the input of Cl− in the groundwater.
In general, the groundwater has a chemical composition within the permissible limits suggested for drinking water, except at some places where the nitrate content exceeds the permissible limit (50 mg/L).
This study adds to our understanding of the mineralization processes of the Dogger groundwater in the Vienne Department and the impact of other influences (agriculture, wastewaters). These findings will be helpful to take measures to prevent spreading of the pollution of this important resource.
NOTES
*Corresponding author.