The Genus Melodinus (Apocynaceae): Chemical and Pharmacological Perspectives ()
1. Introduction
1.1. General Introduction of the Family Apocynaceae Juss. and Genus Melodinus
The genus Melodinus belongs to family Apocynaceae, a large family of a family of flowering plants that includes trees, shrubs, herbs, stem succulents, and vines, commonly called the dogbane family [1] . The family comprises some 1500 species divided over about 424 genera. The former family Asclepiadaceae is included in Apocynaceae according to the APG III system [2] . Members of the family are native to European, Asian, African, Australian and American tropics or subtropics, with some temperate members [1] . Many species are tall trees found in tropical rainforests, but some grow in tropical dry (xeric) environments [3] . There are also perennial herbs from temperate zones [4] . Some are sources of important drugs, such as cardiac glycosides, which affect heart function [5] . These include the Acokanthera, Apocynum, Cerbera, Nerium, Thevetia and Strophantus. Rauvolfia serpentina, or Indian Snakeroot, yields the alkaloids reserpine and rescinnamine, which are useful tools in the treatment of high blood pressure and even some forms of psychosis. Catharanthus roseus yields alkaloids used in treating cancer [6] [7] .
Melodinus clade embraces 109 species which are mainly found in tropical Asia and Oceania [8] .
1.2. Botanical Features of Melodinus
The members of the genus Melodinus consist of stout climber or lianas. Branches dark gray, glabrous; young branch lets and leaves scaly. Petiole 5 mm - 10 mm; leaf blade oblong or narrowly elliptic, 7 cm - 18 cm × 2.5 cm - 5.2 cm, papery, base rounded, apex acuminate; lateral veins 10 - 15 pairs, nearly flat on both surfaces. Cymes umbellate, terminal and axillary, 5 cm - 6.5 cm; peduncle 1.5 cm - 2 cm, glabrous; bracts and bracteoles 3 mm - 7 mm. Pedicel 5 mm - 7 mm, pubescent. Flower buds cylindric, ca. 2 cm, glabrous outside. Sepals broadly ovate, ca. 7 5 mm, ciliate, apex acute. Corolla white, tube ca. 1.2 cm, pubescent inside; lobes oblong, ca. 1.1 cm; corona scales linear, decurrent to lower part of corolla tube, included. Ovary glabrous. Style very short. Berries globose, ca. 10.5 cm in diam [9] -[11] .
This study is an attempt to compile an up-to-date and comprehensive review of the genus Stephania that covers its traditional medicinal uses, chemistry and pharmacology. Many plants of this genus are pharmacologically known but chemically unknown and vice-versa. Therefore, the scope of future research in this aspect is also discussed here.
2. Traditional and Medicinal Uses
Melodinus suaveolens and Melodinus henryi, have been used in Chinese folk medicine for the treatment of meningitis in children and rheumatic heart diseases [11] . They can also “invigorate the circulation of blood”, “stimulate sucking and treat fracture” [12] . It was also used in Chinese folk medicine for the treatment of hernia, infantile malnutrition, dyspepsia and testitis [11] .
Melodinus reticulatus was used to treat cancers as alkaloids were detected in 1983 [13] . Next, several alkalois were detected successively from Melodinus acutiflorus and Melodinus tenuicaudatus [14] [15] .
3. Chemical Constituents
Isolation and structure elucidations of secondary metabolites in Melodinus have been carried out since the 80s [16] . Majority of investigations include the acrial part of the species. To date about 100 compounds have been isolated from 12 species, which are Melodinus acutiflorus F. Muell, Melodinus aeneus Baill, Melodinus balansae Baill, Melodinus celastroides Baill, Melodinus fusiformis Champ. ex Benth, Melodinus guillauminii Boiteau, Melodinus hemsleyanus Diels, Melodinus morsei Tsiang, Melodinus monogynus Roxb, Melodinus scandens J.R. Forst. & G. Forst., Melodinus suaveolens (Hance) Champ. ex Benth, Melodinus yunnanensis Tsiang & P.T. Li.
The isolation and separation technique is very much dependent on the type of fractions. Substances are separated with liquid chromatography using different solvent mixtures with silica gel [13] , charcoal [17] and sephadex [14] [18] . Other types of analytical techniques include thin layer chromatography (TLC) and high performance liquid chromatography (HPLC) [19] -[22] .
The structures are mainly established by mass spectroscopy (MS), ultra-violet spectroscopy (UV), infrared spectroscopy (IR) and 1H and/or 13C nuclear magnetic resonance (NMR). 1H and/or 13C spectroscopy is probably the most useful method in structure elucidation [13] -[15] [17] -[20] [22] -[30] .
Among the secondary metabolites isolated from members of the genus Melodinus are mainly focused on alkaloids (indoles, steroids, diterpenes). The main secondary metabolites isolated so far from the genus Melodinus. consists of 30 - 35 compounds. The profile of all known secondary metabolites of Melodinus as found in literature and their structures are included following.
3.1. Melodinus acutiflorus F. Muell
Rhaxicine 1), rhaximine 2), 16-epi rhazinaline 3), N(l)-methyl-rhazicine 4) [14] [32] .
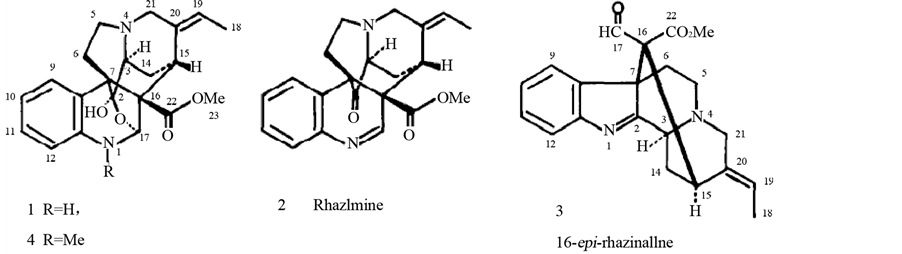
3.2. Melodinus aeneus Baill
(+) vincadifformine (5), (−) tabersonine (6), (−) methoxy-11 tabersonine (7), (−) lochnericine (8), (−) lochneinine (9), (−) ibogamine (10), venoterpine (11), (+) tubotaiwine (12), (+) N-oxy tubotaiwine (13), (+) epi- 16dehydro l4-15 vincamine (14), (+) epi-16dehydro l4-15 vincine (15) [33] .
3.3. Melodinus balansae Baill
Venalstonine dehydro 14-15 (16), venalstonidine epoxy 14-15α (17), paucivenine (18), pleiomutine (19) [34] .

3.4. Melodinus celastroides Baill
(−) tabersoninel (20), leburnamine (21), lisoeburnamine (22), leburnamonine (23) [35] .
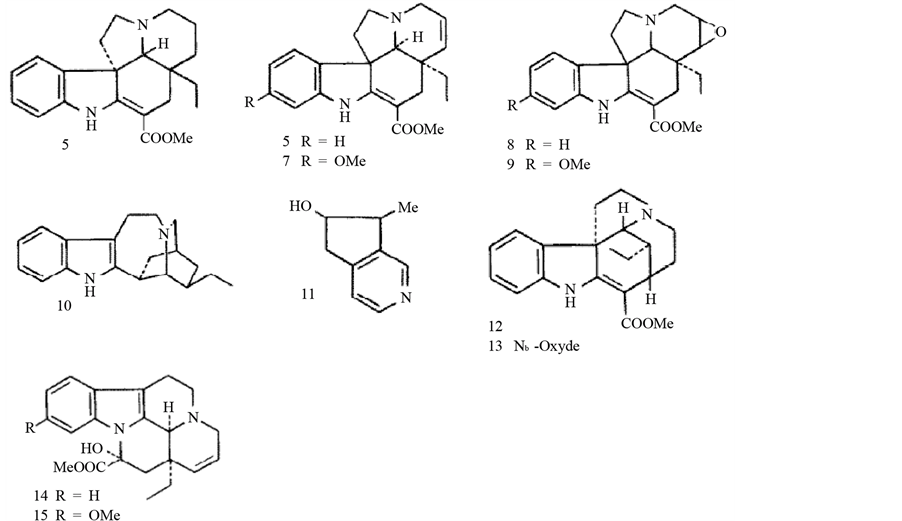
3.5. Melodinus fusiformis Champ. ex Benth
Melofusine I (24), melomorsine I (25), tabersonine (26), 11-hydroxytabersonine (27), 19-acetyltabersonine (28), 11-hydroxy-19-acetyl-tabersonine (29), vincadifformine (30), 11-hydroxyvincadifformine (31), vincadifformineNb-oxide (32), lochnericine (33), 11-hydroxy-14, 15-epoxytabersonine (34), venalstonine (35), venalstonidine (36), 19S-vindolinine (37), 19R-vindolinine (38), eburenine (39), (−)-aspidospermidine (40), (+)-voaphylline (41), (S)-quebrachamine (42), N-acyl-indolinique (43), scandine (44), 10-hydroxyscandine (45), meloscandonine (46), and 19-epi-meloscandonine (47) [20] [26] [30] [36] -[50] .
3.6. Melodinus guillauminii Boiteau
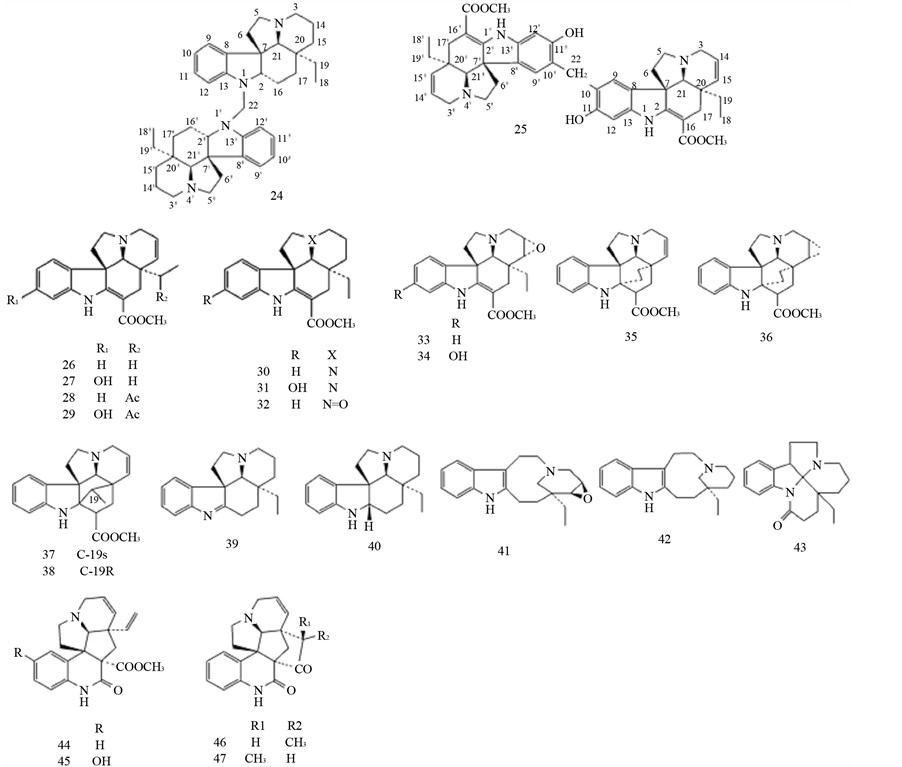
11-hydroxytabersonme (48), venalstomne (49), venalstomdme (50), 14,15-seco-3-oxokopsmal (51), 3-oxovenalstomne (52), 11-methoxy-Δ14-vmcamenme (53), 3-0x0-hydroxykopsmme (54), ll-methoxyd14-vmcanol (55), kopsmme (56), 15-a-hydroxykopsmme (57), 16-epi-Δ14-vmcanol (58), Δ14-vmcanol (59), Δ14-vmcmone (60), Δ14-vmcamenme (61), Δ14-16-Eplvmcme (62), 11-methoxytabersonme (63), 19-β-hydroxyvenalstomne (64), plemcarpamme (65) [51] .

3.7. Melodinus hemsleyanus Diels
Tabersonine (66), 11-methoxytabersonine (67), 11-hydroxytabersonine (68), 11,19R-dihydroxytabersonine (69), ll-hydroxy-14,15-α-epoxytabersonine (70), scandine (71), 10-hydroxyscandine (72), meloscine (73), meloscandonine (74), venalstonine (75), venalstonidine (76), vindolinine (77), picralinal (78), picrinine (79), akuammidine (80), ll-hydroxyvincadifformine (81), 14,15-epoxyscandine (82), 19-epimeloscandonine (83), 16β-hydroxy-19R-vindolinine (84) and 16β-hydroxy-19S-vindolinine (85) [39] .


3.8. Melodinus morsei Tsiang
Melofusine I (24), melomorsine I (25), tabersonine (26), 11-hydroxytabersonine (27), 19-acetyltabersonine (28), 11-hydroxy-19-acetyl-tabersonine (29), vincadifformine (30), 11-hydroxyvincadifformine (31), vincadifformineNb-oxide (32), lochnericine (33), 11-hydroxy-14,15-epoxytabersonine (34), venalstonine (35), venalstonidine (36), 19S-vindolinine (37), 19R-vindolinine (38), eburenine (39), (−)-aspidospermidine (40), (+)-voaphylline (41), (S)-quebrachamine (42), N-acyl-indolinique (43), scandine (44), 10-hydroxyscandine (45), meloscandonine (46), and 19-epi-meloscandonine (47) [17] [20] [26] [30] [36] -[50] [52] .
3.9. Melodinus monogynus Roxb
Medigenin (86), Medinin (87), medigenin acetate (88) [53] .

3.10. Melodinus scandens J.R. Forst. & G. Forst.
9-hydroxyepimeloscine (89); epimeloscine (90), 9-methoxyepimeloscine (91) [27] .

3.11. Melodinus suaveolens (Hance) Champ. ex Benth
Melodinine M (92), Melodinine N (93), Melodinine O (94), Melodinine P (95), Melodinine Q (96), Melodinine R (97), Melodinine S (98), Melodinine T (99), Melodinine U (100), 11-hydroxytabersonine (27), 11-methoxytabersonine (67), tabersonine (26), 19-(R)-acetoxytabersonine (101), 11-methoxy-19-(R)-hydroxytabersonine (102), lochnericine (33), Δ14-vincine (103), vindolinine (77), 11-methoxytabersonine (67), vincadifformine (30), hazuntine (104), 11-hydroxytabersonine (27). 11-methoxyvincadifformine (105), cathovalinine (106), vincoline (107), 19R-hydroxytabersonine (108), 1l-methoxy-19R-hydroxytabersonine (109), 11,19R-dihydroxytabersonine (110), tabersonine (26), suaveolenine (111) [25] [26] [54] .

3.12. Melodinus yunnanensis Tsiang & P.T. Li
Meloyine I (112), 19S-Methoxytubotaiwine N4-oxide (113), 16,19-Epoxy-Δ14-vincanol (114), 14β-Hydroxymeloyunine (115), Meloyunine (116), Δ14-Vincamenine N4-oxide (117), 16β,21β-Epoxy-vincadine (118), 14β,15β-20S-Quebrachamine (119), 3-Oxo-voaphylline (120), 2α,7α-Dihydroxy-dihydrovoaphylline (121) [16] [17] [55] .

4. Biological Activities
In vitro pharmacological activities of Melodinus.
Four species from Melodinus have been studied for their pharmacological activities. Extracts and pure compounds derived from Melodinus were reported to have a concentrate pharmacological activity of antitumor.
4.1. Anticancer Activities
It is known that nature is able to produce a wide variety of chemical entities of novel structure. Many of the new and novel compounds isolated from natural sources might otherwise have never been discovered, especially those of considerable complexity requiring the development of methods for the creation of new ring systems. Natural products appeared to be a promising source for new types of compounds with antitumor activity [56] [57] .
MTT assay is dependent on the reduction of the tetrazolium salt MTT (3-(4,5-dimethylthazol-2-yl)-2,5-diphenyl tetrazolium bromide) by the mitochondrial dehydrogenase of viable cells to form a blue formazan product. The assay measures cell respiration and the amount of formazan produced is proportional to the number of living cells present in culture which will result in lower optical density (OD) [58] .
Anticancer activities were reported in 1998, Demethyltenuicausine (I), a new bisindole alkaloid, was isolated from Melodinus hemsleyanus revealed antitumor activities in pharmacological tests [24] .
Antitumor potential was demonstrated by the indole alkaloids from the leaves and twigs of Melodinus fusiformis and Melodinus tenuicaudatus via the MTT biological assay showed significant cytotoxicity against five human tumor cell lines: SW480, SMMC-7721, HL-60, MCF-7 and A-549 [17] [18] .
Nine isolated from Melodinus suaveolens revealed a positive cytotoxicity with IC50 all around 10 µM against five human cancer cell lines: Five human cancer cell lines, human myeloid leukemia HL-60, hepatocellular carcinoma SMMC-7721, lung cancer A-549, breast cancer MCF-7, and colon cancer SW480 cells [25] .
4.2. Other Biological Activities
A pharmacological test revealed that 11-hydroxyvincadifformine which was isolated from the aerial parts of Melodinus hemsleyanus has significant antifertility activity [19] .
5. Future Perspectives and Conclusions
There is no relative report mentioned the Melodinus products. However, according to its potential, Melodinus, can be published as a high-value export crop for medicinal use.
Although, several research work has been done on some plants of this genus to date, but a large number of species are still chemically and/or pharmacologically unknown. Consequently, a broad field of future research remains possible in which the isolation of new active principles from these species would be of great scientific merit. The alkaloids are of particular interest as many are highly potent bioactive and perhaps responsible for most of activities shown by the plants of this genus. However, the mechanism of their action is still unknown. Hence, a detailed study is required to understand the structure-activity relationship of these constituents. As literature showed, many plant extracts having cytotoxic activity, hence, the particular constituent responsible for the activity may be isolated for further process. In addition, some plant extracts were only screened for their preliminary in vitro activities, so, the advance clinical trial of them deserves to be further investigated. Herein, we described the possible applications in clinical research but further investigations on phytochemical discovery and subsequent screening are needed for opening new opportunities to develop pharmaceuticals based on Stephania constituents.
Acknowledgements
This work was supported by University of Nottingham, Malaysia Campus under the PhD research grant. The authors pay their sincere thanks to editor and referees of the journal, for their valuable suggestions to improve this article. There is no conflict of interest for all authors.
NOTES
*Corresponding author.