Impact of the aqueous extract of dandelion, probiotic and their synbiotic on male lamb’s testicular histopathology relative to semen characteristics ()
KEYWORDS
Dandelion; Histopathology; Probiotic; Ram; Semen; Testis
1. INTRODUCTION
Dandelion (Taraxacum officinale) leaves and roots have been used for hundreds of years to treat liver, gallbladder, kidney, and joint problems. Dandelion is traditionally considered as an alternative and is used for conditions as varied as eczema and cancer [1]. In North America, the Iroquois people prepared infusions and decoctions of the root and whole herb to treat kidney disease, dropsy, and dermatological conditions. As is the case today, dandelion leaves have also been used historically as a diuretic.
In traditional Chinese medicine, it is also acclaimed as a nontoxic herb with exceptional values for its choleretic, diuretic, anti-rheumatic and anti-inflammatory properties. Several flavonoids including caffeic acid, chlorogenic acid, luteolin, and luteolin 7-glucoside have been isolated from the dandelion [2]. Dandelion fresh leaves contain protein, fiber, calcium, phosphorus, iron, potassium, thiamine, riboflavin, vitamin C, and are especially high in vitamin A. The dried leaf is high in potassium (about 4%). Dandelion was included as a diuretic in the US pharmacopeia from 1831 to 1926 [3]. Based on its diuretic effects, dandelion is often included in herbal weight loss and premenstrual syndrome remedies. Some herbalists also recommend dandelion as an herb useful to help prevent atherosclerosis [4]. Others suggest that it may be a useful spring and fall tonic for patients with chronic osteoarthritis and those with a tendency to form gallstones [5]. In addition to its medicinal uses, dandelion serves as a green salad in the gourmet’s garden and as an unwelcome weed in the well-manicured lawn. The leaves are an excellent source of vitamin A. The ground roots are sometimes used as a substitute for chicory roots or coffee beans. The flowers are sometimes fermented into wine. The root is the part most often medicinally used [6]. The German Commission E approves it for use as a diuretic and to treat dyspepsia, liver and gallbladder complaints and appetite loss [7,8]. Recent data on rats revealed that dandelion may have a serious side effect on male fertility. According to an earlier fertility study conducted on mice, plant materials derived from Taraxacum mongolicum and other compositae family pose no risk to fertility [9]. Fertility data specific to Taraxacum officinale are not available. However, recently an aqueous extract of dandelion acts as an anti-fertility agent, as it decreases male rat fertility in vivo. Experimental groups received an orally aqueous extract of dandelion for 60 days in two different sub lethal doses; 1/10 LD50 as high dose and 1/20 LD50 as low dose, whereas the control group received distilled water. The administration of the aqueous extract of Taraxacum officinale resulted in a significant decrease in testis weight in the two experimental groups. Also, distortion of morphology of the seminiferous tubules and arrest in spermatogenesis were observed in the experimental groups [10]. Due to the controversy in this issue, the current study was designed to investigate the effect of administering an aqueous extract of dandelion leaves without/or with cow’s fermented milk probiotic, on the histopathological appearance of ram testes in relation to semen characteristics. The inclusion of probiotic was targeted to ameliorate the adverse consequences on testicular function.
2. MATERIAL AND METHODS
2.1. Animals and Treatments
Twenty four prepuberal Noeimi male lambs aging 2 ±
0.2 months and weighing 20 ± 3.5 kg were raised in the experimental station of Qassim University, central of Saudi Arabia. Animals were offered pelleted ration in addition to green fodder (Trifolium alexandrinum) according to the requirement of the AOAC [11] and have accessibility to clean tap water. Animals were randomly allocated into four treatment groups (n = 6/group). Group 1 animals served as control and orally given 50 ml saline (0.9% NaCl) every other day throughout the experimentation period (8 weeks), group 2 animals were given 50 ml of an aqueous extract of dandelion (6%) as the same schedule in the control, group 3 animals were given 50 ml fermented cow milk probiotic in the same manner and group 4 animals were given 50 ml of the mixture (dandelion: probiotic; 1:1) in the same schedule. Animals were observed for health status and attainment of puberty signs (i.e. the first mount with complete erection).
2.2. Semen Collection and Evaluation
At puberty semen was collected via an artificial vagina and estrous ewe. Ejaculates were collected once a week for ten consecutive weeks. Gross and physical evaluations were done on each ejaculate (i.e. ejaculate volume, sperm concentration, gross motility, individual progressive motility, percent dead sperm, semen pH).
2.3. Blood Collection and Serum Harvesting
Concomitant to the semen collection a venipuncture Jugular blood sample was collected in heparin-free Vacutainer® tube of three animals within a treatment (i.e. total 12 animals) once per week throughout 10 consecutive weeks for the determination of testosterone. Blood samples were cooled for two hours prior to the centrifugation (3000 rpm/30 min/5˚C). Serum was harvested and kept frozen (−20˚C) until further used for testosterone determinations.
2.4. Testosterone Determinations
Testosterone was determined according to Moltz et al. [12] by a commercial ELISA kit (HUMAN Diagnostic Co., Germany). Testosterone determinations sensitivity range was 0.05 - 0.09 ng/ml and intraand inter-assay coefficient of variations were 2.9% and 4.7%, respectively.
2.5. Proximate Analysis of Dandelion Dry Leaves
The chemical composition of dandelion was performed in the biochemistry laboratory and the mean data of analyzing 10 samples of various locations of the crushed powder of dandelion leaves are shown in Table 1.
2.6. Milk and Bacterial Culture
Milk samples were obtained from healthy lactating

Table 1. Chemical composition of dandelion leaves (M ± SEM).
*Mean of 10 samples.
animals located at the experrimental station of the University of Qassim (Al-Qasiim region-Saudi Arabia). Starter cultures of Streptococcus thermophilus, Lactobacillus acidophilus and Bifidobacterium bifidum were obtained from Chr. Hansen’s Laboratory, Copenhagen Denemark.
2.7. Preparation of Probiotic Fermented Milk
Preparation of probiotic fermented milk was carried out according the methods of traditional fermented manufacturing, which carried out using the methods described by [13]. Fresh cow milk have been standardized to achieve about 12% total solid content, and then heated at 85˚C for 15 min, cooled to 40˚C, inoculated with probiotic bacteria (2%) and then incubated for 4 - 8 h at 42˚C. After coagulation, the curd has been tested for pH, then stirred in an electric blender and stored refrigerated (5˚C). The fresh milk was analyzed according to AOAC method [14]. The analysis data of milk were as follow; 2.90 fat, 8.66% solids not fat, 3.24% protein, 4.73% lactose, 0.65% ash, 1.0261 kg/m3 density and −0.408˚C freezing point.
2.8. Preparation of Aqueous Extracts of Dandelion (Taraxacum officinalis)
Dried dandelion (Taraxacum officinalis) leaves and roots weres purchased from local market. Aqueous extract of dandelion (Taraxacum officinalis) containing prebiotic ingredients (prebiotic extract) has been carried out using a method described by Melendeza and Caprilesa [15]. The plant material was pulverized in a grinder. Sixty grams of the pulverized material have been dissolved and extracted with 1000 ml hot distilled water in an electric blender which left running for 15 min. Afterwards, the suspension was left at room temperature for 1 hour, then filtered twice, first through cheese-cloth (50% cotton/50% polyester) and then through filter paper (Whatman No.2). The clear aqueous extract was preserved in sterile dark bottles (500 ml) at −20˚C until further use.
2.9. Preparation of Synbiotic
Synbiotic syrup was prepared by combining equal volumes (v:v) of protective fermented milk with aqueous extract of dandelion (Taraxacum officinalis).
2.10. Statistical Analysis
Data for semen parameters were analyzed by the least square analysis of variances [16]. Differences of means among treatments were compared by the Duncan’s Multiple Range Test (DMRT) [17]. Significance level was considered at P < 0.05.
2.11. Histopathological Examination
Samples of testis were taken, and fixed in 10% neutral buffered formalin for 24 hours. Paraffin sections 6 μm thick were prepared and stained with hematoxylin and eosin (H & E) for the examination of tissue and cellular changes by light microscopy [18,19].
3. RESULTS
3.1. Semen Characteristics
Throughout the entire study we mean by the term “Prebiotic” dandelion leaves aqueous extract.
Semen ejaculate volume (Figure 1) decreased significantly (P < 0.01) in rams given prebiotic (0.92 ml) as compared to control (1.18 ml) rams. Percent of reduction in ejaculate volume was about 22%. However, there were no obvious differences in ejaculate volume among other treatments (1.14 and 1.12 ml for symbiotic and probiotic, respectively).
Sperm concentration (Figure 2) was highest in rams given fermented milk probiotic (3.65 × 109 sperm/ml) than control and other treatments. Taraxacum officinale aqueous solution slightly (P > 0.05) decreased the sperm count (2.57 × 109 sperm/ml) by about 28% of the control value (2.8 × 109 sperm/ml).Combining the fermented milk with the dandelion extract (i.e. synbiotic) ameliorated this effect (2.76 × 109 sperm/ml).
Sperm individual progressive motility (Figure 3) was reduced (P < 0.001) in rams administered Taraxacum officinale (47.2%) as compared to control (54.2%), this represent about 15% decrease. On the other hand, probiotic significantly (P < 0.001) increased sperm motility than control and other treatments (63.0% and 50.3% for probiotic and synbiotic rams, respectively). The increase that has been gained by drinking the fermented milk on sperm motility reached 16% above the control rams. Combining fermented milk with the dandelion did not enhance its effect on sperm motility.
Furthermore, prebiotic (22.4%) and synbiotic (26.4%) increased (P < 0.001) percentage of dead sperm (Figure 4) as compared to control (17.1%), however rams given probiotic gave ejaculates with the lowest percent of dead sperm (14.7%). The difference due to probiotic treatment on percent dead sperm was not statistically significant.

Figure 1. Effects of prebiotic, probiotic and synbiotic on ram ejaculate volume.

Figure 2. Effects of prebiotic, probiotic and synbiotic on the ram sperm concentration.

Figure 3. Effects of prebiotic, probiotic and synbiotic on the percentage of ram progressive motile sperm.
Semen pH value (Figure 5) was not statistically (P > 0.05) different among treatments, even though administration of Taraxacum officinale decreased the pH value to be more acidic (6.14), however pH value of the control semen was 6.86, close to neutral.
Percent of abnormal sperm (Figure 6) was not different (P > 0.05) among treatments (range, 6.6% - 7.1%).
3.2. Blood Testosterone
There found significant differences (P < 0.01) among treatments on serum testosterone (5.36, 4.33, 6.31 and

Figure 4. Effects of prebiotic, probiotic and synbiotic on the ram percentage of dead sperm.

Figure 5. Effects of prebiotic, probiotic and synbiotic on the ram semen pH value.

Figure 6. Effects of prebiotic, probiotic and synbiotic on percentage of abnormal sperm of ram semen.
5.73 ng/ml, for control, pre-, proand synbiotic, respectively). Dandelion extract significantly decreased peripheral testosterone concentration by about 19% than in control; however probiotic increased testosterone by about 18%. Even though, synbiotic showed a slight elevation in testosterone, but not significant (Figure 7).
3.3. Histopathology of Testes
Histopathological changes in the testis sections stained with H&E were evaluated by light microscopy. A summary of the evaluations is shown in Table 2. There found

Figure 7. Effects of prebiotic, probiotic and synbiotic on peripheral testosterone of rams.

Table 2. Histopathological changes in lamb’s testes as influenced by dandelion, probiotic and their synbiotic mixture.
+++ = Severe, ++ = Moderate, + = Positive, – = Négative/Normal/ Null.
localized edema and vaculations in the interstitial tissue of testis in the dandelion extract- (Photo 1) and synbiotic- (Photo 2) treated lambs. Less edema and vaculations were observed in probiotic-treated lamb testes (Photo 3). The dandelion extract-treated lambs also showed more histo-architecture disturbance of seminiferous tubules in testes (Photo 1) compared to probiotic- (Photo 3) and synbiotic- (Photo 4) treated lambs. These sections showed less increased cellular density and more cell death in the seminiferous tubules germinal cells of dandeliontreated compared to probiotic, synbiotic-treated and control lambs (Photo 4).
4. DISCUSSION
Dandelion is a biennial herbaceous plant native to temperate areas, and it contains large amounts of inulin (12% - 15%) and oligofructans in its tap roots [20,21]. Dandelion was found to have several medicinal benefits [22-24]. Dandelion compounds increase bile production and flow. The increased bile flow might improve cholesterol metabolism [24]. The leaves are used to help digestion, and as diuretic. Dandelion is promoted as a blood purifier. Dandelion root has been used to detoxify the liver and gallbladder, while its leaves used to support

Photo 1. A cross section in a lamb testis given dandelion extract (prebiotic) showing testes histo-architecture disturbance seminiferous tubules (thin arrows), interstitial tissue edema and vaculation (thick arrows) (×200).

Photo 2. A cross section in a lamb (#78) testis given synbiotic showing testes histo-architecture disturbance seminiferous tubules, interstitial tissue edema (thick arrows) (×200).

Photo 3. A cross section in a lamb (#70) testis given cow milk probiotic showing interstitial tissue edema and vaculations (thick arrows) (×200).
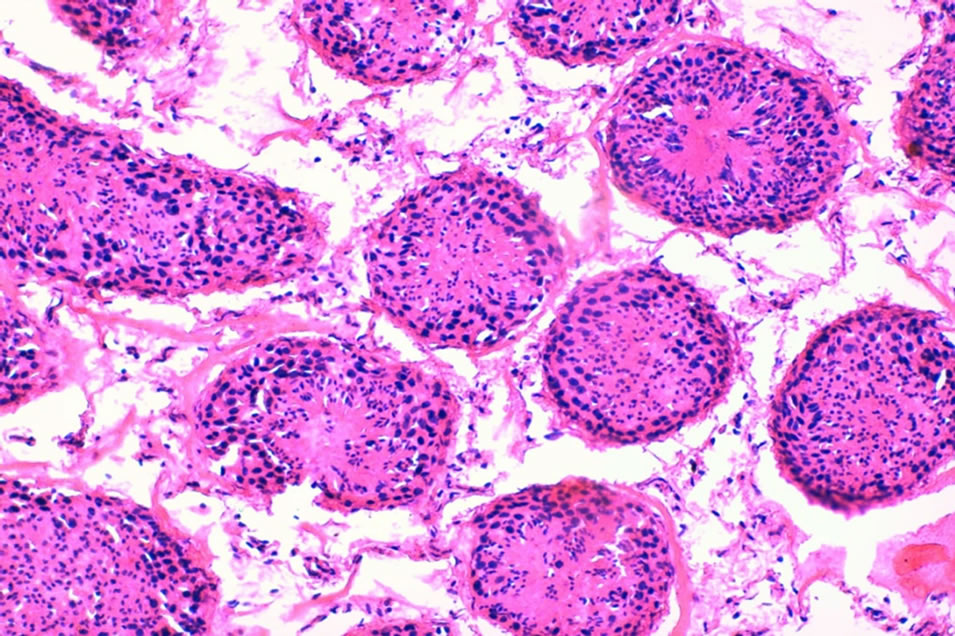
Photo 4. A cross section in a control lamb (#48-control) testis showing normal histo-architecture seminiferous tubules, and interstitial tissue (×200).
kidney function [25]. There have been well established reports of the significance of dandelion on hepatic cell function to synthesize the bile salts which are of relevance to human suffering liver cirrhosis. Above its effect on liver and bile secretion there exist a belief among public that dandelion enhances the aphrodisiac properties in males. Due to the lack of studies on the effect of dandelion on male reproductive performance in general and on farm animals male fertility in specific, this study was implemented to monitor these effects in ruminant animals since some pastures contain scattered plants of this weed. The testicular structure damage due to receiving dandelion aqueous extract might be due to its richness in alkaloids which might deteriorated the spermatogenesis within seminiferous tubules (Photo 1).
Leydig cells in the interstitium of testis are the primary source of testosterone, which stimulates the differentiation of the male phenotype and spermatogenesis in the testes [26,27]. Loss of Leydig cells significantly contributes to the age-related decline of serum testosterone [28-30]. Substantial losses in serum testosterone may result in the inactivation of spermatogenesis. In a recent study in rats [10] similar findings were obtained (i.e. reduced sperm count, low testosterone). They attributed these adverse effects of dandelion extract on sperm count and quality to the significant decrease of androgen receptors in seminiferous tubules. There found similar effects when Echinops echinatus roots (compositae) was given to male rats and these adverse effects on sperm and male hormone was attributed to the terpenoid content in the aqueous extract which exists in dandelion [31]. The decrease in sperm motility might be caused by a decrease in AT Pase activity which might be linked to defects in the ultrastructure of sperm [32].
However, in a study in mice there found a beneficial effect of dandelion extract administration by up regulating estrogen, progesterone and follicle stimulating hormone receptors in females [33]. Moreover, it is unknown the cause-result relationship of the significant decline in testosterone accompanied with the decline in sperm count and percentage of progressive motility. It is well known that androgens are essential for normal spermatogenesis and male fertility, but how androgens exert this effect remains uncertain. Androgen receptors (ARs) are expressed in several testicular cell types, but continuing uncertainty exists over which cell type mediates androgen control of spermatogenesis. Androgen signaling via Sertoli cells (SCs) is essential for complete spermatogenesis [34].
The present results were parallel to those in rats, who found that the testis cellular and histology structure is affected by toxicity of administration of the aqueous extract of dandelion (Taraxacum officinale) [10]. In the present study, cellular and tissue changes were noticed in the dandelion-treated lambs, including generalized and localized edema interstitial tissue. Edema is a clear sign of the toxic effect of aqueous extract of dandelion or increased permeability of interstitial blood vessels. The dandelion-treated animals also showed testes histo-architecture disturbance of seminiferous tubules which could proceed to edema. These dandelion animals also showed testicular cell death signs which might be due to apoptosis or arrest in spermatogenesis. In the lambs fed probiotic alone or in combination with dandelion, the histological picture was improved than in dandelion. The signs of improvements were decreases in the histo-architecture disturbance of seminiferous tubules and increased cellular density of seminiferous tubules. This useful effect could be due to antioxidative stress effects or the synergizing effects on the spermatogenesis due to probiotic components [2-4,35]. It has been found that probiotic bacteria exert an antioxidant effect on sperm to protect against the surrounding free radicals [36]. This would explain the less deteriorative effect of dandelion extract when mixed with probiotic (i.e. synbiotic) in our study. The adverse effect of dandelion and the ameliorative effect of probiotic on spermatogenesis and testosterone production wouldn’t vary between rats (monogastrics) and rams (ruminants).
5. CONCLUSION
Ingesting dandelion (Taraxacum officinale) by males for curing constipation, hepatic and gall bladder malfunction or even for treating joints disorders must be cautioned not overconsumption to avoid the adverse effects on their fertility. Co-administration of probiotic fermented cow’s milk alleviated the adverse effect of water extract of dandelion leaves on testes. Male animals grazing on a pasture containing this herb scattered within the forage crop must be hand removed. Also, a multidisciplinary study must be advanced on purifying the various constituents of dandelion as to test each single component on the testis functions.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the technical support of Mr. Said Yusuf.