1. Introduction
Piper aduncun L. (Piperaceae), popularly known as monkey-pepper and long-pepper, is a pioneer shrub, native in Americas—Brazil, Isfoundin Acre, Pará, Mato Grosso, Ceará, Bahia, Minas Gerais, Espirito Santo, Rio de Janeiro, São Paulo and Paraná States [1,2]. Although it is not cultivated commercially, the species has a great potential because of its antimicrobial property, insecticide and essential oil of low toxicity, making them highly valuable for pharmaceutical and chemical industry [3-6]. The search for vegetables that exhibit antimicrobial characteristics has increased in recent years; however, most studies focus solely on the essential oil produced by some medicinal species. The studies of agronomic areas are scarce and limited to a very restricted number of species.
Moreover, cultivation in farming systems, with either economic or conservational purpose, requires a lot of care which depends on the previous knowledge of the physiological and ecological requirements at various stages of vegetative cycle [7]. So, the cultivation of medicinal plants in different environmental conditions is an important and attractive theme for agronomic research, which gives incipient knowledge of the responses of these species in protecting environment.
The radiation in terms of quality and quantity, is surely one of the most important factors in the physical environment, which can affect the morphology and physiology in plants [8,9]. High light intensities, in general, may promote a decrease in photosynthetic efficiency and result in degradation of photosynthetic apparatus [10]. Lima Junior et al. [11], working with Cupaniavernalis, observed changes in light intensity to promote morphological alterations, affecting CO2 assimilation and transpiration directly, and promoting changes in net photosynthesis. Nevertheless, plants have answers diversified in relation to this environmental factor and most of the studies do not evaluate physiological variables directly, especially those who involved gas exchange, in order to check physiological responses [12].
Adjustments in photosynthetic apparatus determine the adaptive plasticity of species to different irradiance conditions. According to Alvarenga et al. [13], plants with larger efficiency in conversion of radiant energy into chemical energy, achieve more performance and productivity. In this way, studies on gas exchange may be one way to verify the best condition of radiation, which enables greater essential oil and biomass yields in medicinal plants. According to Zavala [14], there are positive correlations between CO2 assimilation rates and growth and the secondary metabolism.
The aim of this study was to evaluate the effect of irradiance spectrum in gas exchange and production of photosynthetic pigments of Piper aduncum, considering their medicinal importance and lack of knowledge of the adaptive capacity in photosynthetic process at different ecoambientes.
2. Material and Methods
2.1. Experiment Site
The experiment was conducted between April and August of 2012, at Gota da Esperança Farm, belonging to the Department of Agriculture (DAG), in Federal University of Lavras, with the following geographical coordinates: 21˚14'07"S and 44˚58'22"W, at 879 maltitude. The averages of climatic conditions observed during the experiment were provided by Climatological Station of the Department of Agricultural Engineering of UFLA, had maximum temperature of 25.06˚C and a minimum of 13.54˚C, precipitation of 1.28 mm and a relative humidity of 72.7%.
2.2. Plants and Cultivation
Seeds from matrix plants were pre-germinated in Petri dishes on three sheets of filter paper and kept in a growth chamber at 25˚C and under a photoperiod of 12 hours, for 30 days. After this period, the seedlings were transferred to polystyrene trays with 72 cells filled with substrate Plantmax® and kept in greenhouse with 50% of shade, until they reach 2.5 cm. After reaching 2.5 cm, the plants, even in the trays, were acclimatized for 14 days before planting in their respective treatments, irrigated daily, once for the plants for cultivation in full sun (100% radiation) were previously acclimatized for 7 days at 70% of irradiance and subsequently for 7 days in full sun before the final planting.
After acclimatization, the seedlings were transplanted to plastic pots 6 liters, containing substrate composed of subsoil, sand and cattle manure in the proportion of 2:1:1, and disposed in different irradiance treatments. The physico-chemical characteristics of the soil were analyzed by the Laboratory Analysis of Soils of Federal University of Lavrasand were: pH: 5.4, P: 4.13 mg∙dm−3; K: 73.32 mg∙dm−3, Ca: 2.30 cmolc∙dm−3, Mg: 0.30 cmolc∙dm−3, Al: 0.10 cmolc∙dm−3, H + Al: 2.90 cmolc∙ dm−3, V: 49.00%; organic matter: 2.10 dag∙kg−1, Clay: 70.00 dag∙kg−1; Silt: 16.00 dag∙kg−1 and Sand: 14.00 dag∙kg−1.
2.3. Variables Analyzed
At five months of growth, gas exchange were evaluated, selected at random, two fully expanded leaves, located between the second and third node in five plants per treatment. The variables analyzed were: net photosynthesis (A), stomatal conductance (gs), transpiration (E), intercellular CO2 concentration (Ci), vapor pressure deficit (VPD) and leaf temperature (TF). In addition, were calculated ratio Ci/Ca, carboxylation efficiency (A/Ci), and the instantaneous efficiency of use of water (A/E).
The measurements were conducted at 9 am, without cloudiness, with portable CO2 infrared analyzer (LI-6400, LI-COR®), which was operated system with artificial source of photosynthetically active radiation (Blue + Red LED LI-6400-02B, LI-COR®, Lincoln, USA). The density of photosynthetically active photon flux density (PPFD) was 900 mol m−2∙s−1 treatment with 50% irradiance and the colored nets, 1200 µmol m−2∙s−1 and treating with 70% of irradiance and 2000 µmol m−2∙s−1 for the treatment of irradiance at 100%, which was standardized according to the ambient radiation incident on each day of analysis. The CO2 concentration atmosphere in the chamber was 380 ± 3 µmol−1mol CO2.
The photosynthetic pigments analyzed chlorophyll a, chlorophyll b, a/b ratio, total chlorophyll and carotenoids. The extraction was performed according to the methodology reported by Lichtenthaler and Buschmann [15], being collected fully expanded leaves located at the third node, five months after transplantation. After collection, leaves were placed in aluminum foil and transported in polystyrene boxes containing ice for immediate extraction and quantification of pigments.
For extraction of the pigments weighed 200 mg of fresh leaves and homogenized with 10 mL of 80% acetone (v/v), filtered through glass wool, completing the volume for 30 mL 80% acetone. Immediately following this procedure was carried the reading of the absorbance at 663.2 nm, 646.8 nm and 470 nm. All procedures were done in the dark, to preserve the integrity of pigments. The entire procedure was performed in the dark to prevent degradation of chlorophylls. The content of chlorophyll a, b and carotenoids were calculated using the Equations (1)-(3) respectively, and expressed in mg g−1 fresh weight
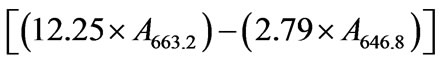 (1)
(1)
 (2)
(2)
 (3)
(3)
After determining the contents, were done relations chlorophyll a/b and total chlorophylls, summing the contents of chlorophyll a and b.
2.4. Treatments
The treatments were characterized by cultivation of plants under five spectral irradiance produced by Sombrite® (30% and 50%), two Chromatinet® nets in red (R) and blue (B) colors, which block 50% of incident radiation, and one treatment at full sun. Irrigation was performed daily, keeping the soil at field capacity.
With the assistance of a portable spectroradiometer (USB-650 Red Tide) coupled to source of electromagnetic radiation DT-MINI (200 to 2000 nm) and a probe reflectance R400-7-VIS-NIR (US BioSolutions Ocean Optics®) we evaluated the radiation spectrum of different environments, with a spectral resolution of 1 nm. The irradiance normalized for each environment presented higher values in terms of quantity and size of the spectrum, for the environment with 100% irradiance, followed by the environment R, 70% and 50% and the B environment (Figure 1). Note, that the blue net gave a peak of irradiance between 450 - 550 nm and red net between 490 - 690 nm.
2.5. Statistics
The experimental design was completely randomized, and the observed data submitted to the Lilliefors normal-

Figure 1. Normalized irradiance for wavelength radiation in five environments: 50%, 70% and 100% of irradiance, R (red net) and B (blue net).
ity test at 5% significance and submitted to analysis of variance, the means compared by the Scott-Knott test at 5% of probability, by the program SAEG [16].
3. Results and Discussion
Significant differences were found among treatments in the gas exchanges’ variables which were evaluated (Figure 2). The net photosynthesis was superior in the treatment with 100% irradiance, and lower in plants cultivated under colored nets. Variations in the spectral irradiance stimulate rapid responses in photosynthetic activity and the increase in brightness provides an increase in photosynthesis until they reach the saturation point [17]. Therefore, the rise in photosynthesis observed at treatments with higher irradiances might have supplied better conditions for CO2 assimilation allowing greater photosynthetic activity. According to Souza et al. [18], plants growing under high radiation might develop mesophyll cells with more chloroplasts, enabling greater photosynthetic activity.
The low net photosynthesis observed in plants grown under red nets may be due to low chlorophyll content, low carboxylation efficiency and CO2 assimilation showed in this treatment (Figure 3). Furthermore, the low irradiance (50%) afforded this treatment resulted in lower photosynthetic activity, as observed in in vitro studies [19].
In another study, it was observed that blue light has an important role in regulating the parameters of photosynthesis [20]. However, the balance between the red and blue is more important than the concentration of these bands in the spectrum resulting from such colored nets [21]. Similar results were observed for Capsicum annuum grown under white, yellow and red light, where despite of blocking 35% of the radiation, the higher photosynthetic rate was observed under white light [21].
The stomatic conductance presented a variable response in relation to irradiance conditions, which was higher in treatments with 100%, 50% and red net. Furthermore, plants grown under red nets showed higher values of Ci, which are lower in treatments with 70% radiation and blue net. The higher stomatal conductance observed in red net may have been answerable for the greater Ci observed in this treatment.
These results demonstrate that photosynthetic activity observed in treatment at 100% of irradiance may have been afforded thru higher stomatal conductance observed under these conditions. Furthermore, the low photosynthetic activity observed in plants grown with 50% of the radiation and red net can be attributed to other factors, not the internal CO2 concentration, although this relationship was observed for plants grown in 70% of radiation and blue net. Studies with Vaccinium corymbosum at

Figure 2. (a) Photosynthetic activity; (b) Stomatal conductance; (c) Rate of perspiration; (d) Concentration of intracellular of CO2; (e) vapor pressured eficit; (f) Leaf temperature of Piper aduncun grown under different spectral irradiance—50% of irradiance, 70% of irradiance, 100% of irradiance, R (red net) and B (blue net). *Means followed by the same letter do not differ by Scott-Knot test at 5% probability.
different shading levels (40%, 50% and 75% of shading) do not show changes in stomatal conductance [22]. Furthermore, stomatal conductance affected by different levels of irradiation has already been reported in the literature [23]. The treatments with 70% and 100% of irradiation were presented with the highest rates of transpiration, leaf temperature and vapor pressure deficit.
In this way, we can infer that the high irradiance might favor the process of photorespiration. However, as the plants were grown at field capacity, i.e. not suffering water stress, this process may not have occurred, while the losses occurring in dry matter or its partition were due to the low efficiencies of water use observed this condition, which necessitates the further studies to check their productivity in such situations.
Effect of high irradiance favoring the process of photorespiration was documented in another study, where plants were not kept on field capacity of the soil [24]. The low transpiration observed in treatments with colored nets and 50% of radiation is considered as a strategy to reduce losses of carbon under this condition. As observed by Yang et al. [25], the effect of different light intensity in photosynthesis was found.
High transpiration, leaf temperature and vapor pressure deficit found in treatment with 100% of irradiance associated with a high stomatal conductance can indicate that the species does not have a strong regulatory mechanism to open and close the stomata. Furthermore, it showed a decrease in the photosynthetic process in plants grown under low-radiation and colored net conditions and gave a lower water loss and leaf temperature. Thus, nets can have promoted a reduction in photorespiration and stabilized the photosynthesis [22].
The relation of Ci/Ca indirectly shows limitations in photosynthesis caused for decrease in acquisition of a non-stomatal CO2. Given that the external concentration of CO2 is constant (Ca), the increase in Ci/Ca is due solely to changes in the internal concentration.
If Ci is increasing, it means that the CO2 present in mesophyll cells is not being set during carboxilative possibly for damage sustained in its structure reducing net photosynthesis. With this manner, it can be seen that the greatest ratio Ci/Ca has been found in plants grown with 50% of brightness and in red net. Moreover, the lowest

Figure 3. (a) Intra/extra celular CO2; (b) Carboxylation eficience; (c) Water use efficiency. 50% of irradiance, 70%, of irradiance, 100% of irradiance, R (red net) and B (blue net). *Means followed by the same letter do not differ by ScottKnot test at 5% probability.
ratios were observed in treatments with 70% and 100% luminosity. These results are in accordance with the photosynthetic rates found in these treatments, indicating plants grown in treatments with 70% and 100% of brightness were those with lower relations Ci/Ca owing to CO2 consumed in the carboxylation process.
The A/Ci ratios were higher in plants grown under 100% of irradiance and lower under 50% and colored nets (Figure 3). In this way, it can be suggested that photosynthetic activity and carboxylation efficiency are more related to the availability of radiation than spectral quality. It can be seen that colored nets had a blockage of radiation equivalent to 50%. The A/E ratio showing us the instantaneous efficiency of water use was higher in treatment with 50% of radiation and lower in treatments with 70% and 100%. This ratio can indicate the efficiency of plants using water, representing photosynthetic properties at a given transpiration rate [26].
Thus, it was observed that plants cultivated under 100% of irradiance though showed the highest photo-synthetic rates. They had a great loss of water through transpiration, which reduced their water use efficiency. Additionally, this parameter can be used to denote the adaptability of plants to environmental changes [27].
When compared with the plants grown in 50% of irradiance, there were lower transpiration rates and higher efficient use of water, which may indicate that the plant has the ability to maintain a certain level of photo-synthesis and carbon assimilation. The greater efficiency of water use in plants grown under partial shade was also observed in Illicium lanceolatum [27].
The plants grown under full sun have higher rates photosynthesis associated with high perspiration, high leaf temperatures and low efficiency of water use, which can promote a lower optimization in production of carbonaceous compounds in relation to water loss. The leaves of Piper aduncun had higher quantity of total and chlorophyll a in treatments under 70% of irradiance. Chlorophyll b levels were changed due to the variation in irradiance spectrum, where the quality of radiation damaged the pigment production (Figure 4).
The ratio of chlorophyll a/b was also changed for the treatment being superior in 70% of irradiance.
The increment of chlorophyll b in environments with a reduced irradiance spectrum is an important characteristic for adaptation to the shaded, because this pigment captures photons in longer wavelengths observed in this type of environment [28]. So, it can be inferred that species studies do no present adaptation in environments with low levels of irradiance because the increasing levels of chlorophyll b in fully radiation. This result is consistent with the observed gas exchange variables.
There was a greater photosynthetic activity in the treatment with 100% followed by 70% of irradiance, indicating an enlargement of absorption spectra even under conditions of high light intensities. While, these characteristics are in accordance with classification of ecological pioneer from this species, as seen sometimes considered a weed [29,30].
The content of chlorophyll a, b and total observed in treatment with 70% of irradiance indicates that it can adapt this luminous condition. In addition, the greatest amount of pigments can show a high chlorophyll synthesis and a slower degradation of chlorophyll b, relative to chlorophyll a balance between the photosystems [31]. The pigments content found in plants grown under red net might be related to smallest relationship red: far red (R/FR) [18]. Moreover, lows levels observed in leaves which have grown in blue net are at odds with the literature, given that, the blue light affects the biosynthesis of

Figure 4. Content of pigments: (a) chlorophyll a; (b) chlorophyll b; (c) Ratio chlorophyll a/b; (d) Total chlorophyll; (e) Carotenoids. 50% of irradiance, 70%, of irradiance, 100% of irradiance, R (red net) and B (blue net). *Means followed by the same letter do not differ by Scott-Knot test at 5% probability.
chlorophyll and other pigments by genetic regulation [32]
According to Larcher [33], changes in chlorophyll biosynthesis thru changes in the spectral quality could provide advantages for growth and reproduction of plants. So, these results indicate that long pepper does not adapt chromatically to progress its photosynthetic performance. On the other hand, an increased level of chlorophyll in plants grown under blue net has been observed for other medicinal plants, such as: Catharanthus roseus [34], Ocimum gratissimum [35] and Mikania laevigata [18].
The higher content of carotenoid was seen in treatment with 100% and lower irradiance in the colored net. Carotenoids are pigments that act as sunscreens by rapid quenching of excited states of chlorophyll protecting it from oxidation [36]. In this way, high levels of carotenoids in plants under 100% of radiation are associated with a sufficient protection of the receptor complexes of photosystems light, proving the high photosynthetic activity observed in this work. High levels of carotenoids in plants grown under full sunlight were observed in Artemisia vulgaris [37].
From the data obtained, it can be observed that Piper aduncun develops different mechanisms for adaptation to different irradiance conditions. Moreover, it can be inferred that the photosynthetic process and production of pigments are more efficient at light intensities and wider spectra, showing higher photosynthetic activity when grown under 100% and 70% irradiation.
However, to remain under these conditions of light, a high-water availability is necessary, since the efficiency of water usage is reduced due to the high-temperature leaf.
It is noteworthy that studies are needed to verify the production and translocation of assimilating these different conditions of radiation, aimed at increased production of essential oil.
4. Acknowledgements
The authors thank FAPEMIG, CNPq and CAPES for the granting of scholarships and financial aid to perform the work.
NOTES