Peripheral tolerance of antigen-specific Th cells induced with polyethylene glycol-conjugate of protein antigen ()
1. INTRODUCTION
Polyethylene glycol (PEG) is a linear synthetic polymer produced by the polymerization of ethylene oxide. PEG has repeating units of ethylene oxide groups that are linked by flexible ether bonds, and is uniquely soluble in both water and organic solvents [1]. PEG is safe for internal consumption, and extremely stable and nonimmunogenic when introduced into the circulation [2]. These features of PEG have enabled the development of PEGprotein conjugates as therapeutic drugs [3-6]. It has long been known that protein antigens conjugated with PEG serve as immunological tolerogens when administered to experimental animals. It is nearly 40 years since Lee and Sehon [7,8] first reported the tolerogenic capacity of hen egg albumin (ovalbumin: OVA) coupled with polyethylene glycol. In these pioneering studies, it was demonstrated that immune tolerance was established on carrier (OVA)-specific Th cells. Based on the results of these early studies, various PEG-allergen conjugates were tested for their efficacy in treating patients with allergic diseases [9,10]. To date, however, none of the PEG-protein antigen conjugates has been used effectively as a tool for antigen-specific immune intervention. We studied the response and differentiation of OVA-specific naïve Th cells upon exposure to tolerogenic PEG-OVA with the hope that such knowledge may lead to practical application of PEG-protein antigen conjugates to induce immune tolerance.
2. MATERIALS AND METHODS
2.1. Mice
C57BL/6 mice, 2 months old, were obtained from Sankyo Laboratory Service (Tokyo, Japan) and were maintained in the animal-care facility of Toin University of Yokohama. C57BL/6-TG(TcraTcrb)425-CbnJ (OT-II) mice [11] were obtained from the Jackson Laboratory (Sacrament, CA). This study was reviewed and approved by the research ethics committee of Toin University of Yokohama.
2.2. PEG-OVA
Polyethylene glycol conjugate of OVA (PEG-OVA) was prepared by the method described by Saito et al. [12]. Five milligrams of OVA (albumin from chicken egg white, grade VII; Sigma-Aldrich, St. Louis, MO) were dissolved in one ml of 0.1 M sodium tetraborate (pH 10.0), and then 1,200 mg of activated PEG2 [2, 4-bis(Omethoxypolyethylene glycol)-6-chrolo-s-triazine] [13] (Seikagaku Kogyo, Ltd., Tokyo, Japan) was added to the solution. The reaction mixture was incubated under stirring at 37˚C for one hour. After the reaction, the mixture was diluted with 10 ml of 0.1 M borate buffer (pH 8.0). The unreacted polyethylene glycol was removed by extensive ultrafiltration using a PM-30 membrane (Merck Millipore, Darmstadt, Germany). Protein concentrations were measured by the Biuret method [14]. In the PEGOVA thus prepared, the average degree of modification of 22 free amino groups in an OVA molecule was 54%, as measured by the colorimetric titration of the free amino groups using trinitrobenzene sulfonate [15].
2.3. Tolerance Induction
Female C57BL/6 mice, 3 months old, received intraperitoneal injections of PEG-OVA (100 μg in 200 μl of PBS) or PBS (200 μl) at weeks 0, 1 and 2. Subsequently, these mice were immunized with OVA (250 μg in 200 μl of PBS) at weeks 4, 5, 6 and 11. At week 13, blood samples were obtained from orbital venous plexus using a capillary tube, and were allowed to clot at 4˚C. Sera were stored at −20˚C until analysis. Levels of IgG class antiOVA antibodies were measured by ELISA.
2.4. Proliferative Resonse of OVA-Specific Th Cells in Vivo
Splenic lymphocytes from OT-II mice were labeled with carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes Inc., Eugene, OR) by the method described by Quah et al. [16]. Briefly, splenic lymphocytes were isolated by density-gradient centrifugation using NycoPrep reagent (Axis-Shield PoC; AS, Oslo, Norway). Four μl of CFSE solution in dioxane (2.5 mM) was added to 2 ml of the lymphocyte suspension (107/ml in PBS containing 2% FBS), and the solution was reacted at room temperature for 5 minutes. After washing with PBS containing 2% FCS, 107 labeled cells were resuspended in 500 μl of PBS containing 2% FCS, and were transferred into the circulation of histocompatible C57BL/6 mice via the tail vein. The fluorescence at 535 nm of the CD4+ OT-II Th cells was measured by a FACS Vantage Immunocytometry System (Becton Dickinson, San Jose, CA).
2.5. Stimulation of OVA-Specific Th Cells with Antigenic Peptide in Vitro
Spleen cells from OT-II mice were suspended at 5 × 106 cells/ml in RPMI 1640 medium containing 50 μM of 2-mercaptoethanol, 100 units/ml of penicillin G (potassium salt), 100 μg/ml of streptomycin sulfate, 250 ng/ml of amphotericin B, and 10% FCS. Aliquots (200 μl) of the cell suspension were distributed in wells of a polystyrene flat-bottomed plate, and then various concentrations of OVA323-339 peptide were added [17]. The plate was incubated at 37˚C under 5% CO2 for 24 hours. Incorporation of bromodeoxyuridine (BrdU) was tested during the last 8 hours of the incubation by adding BrdU to each well to obtain a final concentration of 100 μM. After incubation, the supernatant was carefully removed by aspiration. Subsequently, the cells were fixed to the plate by treatment with methanol and drying. Incorporated BrdU in the genomic DNA of the cells was measured by ELISA with anti-BrdU monoclonal antibody [18].
2.6. Enzyme Immunoassay for OVA-Specific Antibodies
Levels of serum IgG class anti-OVA antibodies were measured by ELISA [19]. Each well of a polystyrene flat-bottomed plate (Nunc-Immuno Plate 442404; Nalge Nunc International, Roskilde, Denmark) was coated with 1 μg of OVA in 0.1 ml PBS at 4˚C overnight, blocked with 3% BSA in PBS for one hour, and reacted with diluted serum (50 μl) at 4˚C for one hour. After 6 washings with PBS containing 0.05% Tween 20, 50 μl of peroxidase-conjugated goat anti-mouse IgG antibodies was added to each well, and the plate was incubated at room temperature for 90 minutes. After extensive washing, 100 μl of 4.8 mM o-phenylenediamine (Wako Pure Chemical Industries, Ltd., Osaka, Japan) in 0.1 M citrate-phosphate buffer (pH 5.0) and hydrogen peroxide (2.6 mM) was added to each well. After incubation at room temperature for 5 minutes, the reaction was terminated by adding 50 μl of 4 N H2SO4. The absorbance at 490 nm was measured by a model 550 Plate Reader (Bio-Rad Laboratories, Hercules, CA).
2.7. Cytokine Gene Expression
The levels of transcription of the cytokine genes were measured by real-time PCR analysis. Spleen lymphocytes (4 × 107) of the OT-II mice were stimulated in vitro with 2.2 μM of OVA323-339 peptide in 8.0 ml of RPMI medium supplemented with 10% FCS and 50 μM of 2-mercaptoethanol. After 16 hours, the cells were harvested and lysed with Trisol Reagent [20]. Cytoplasmic RNA was extracted as described in the manufacturer’s instructions, and was further purified by using an RNAfree DNase in the presence of recombinant ribonuclease inhibitor (Takara Shuzo, Shiga, Japan). cDNA molecules were synthesized by using a reverse transcriptase and random hexamer, and were amplified by Taq thermostable DNA polymerase in the presence of SYBR Green [21]. The quantitative real-time PCR analysis was conducted by using a Roche Lightcycler 480 (Roche Diagnostics GmbH, Mannheim, Germany). The nucleotide sequences of the primers used for the analysis were as follows: for Mouse IL-2 [22], AACTGAAA CTCCCCAGGAT and AGGGCTTGTGAGATGATGC; for Mouse IL-4 [23], CCTCACAGCAACGAAGAACA and AAGTTAAAGCATGGTGGCTCA; for Mouse interferon γ (IFN-γ) [24], AAGCAATCAGGCCATCAGC and ATCAGCAGCGACTCCTTTTC; for Mouse IL-10 [25], CCAAGCCTTATCGGAAATGA and TGGCCT GTGACACCTTGG; and for Mouse IL-17 [26], TCTCTGATGCTGTTGCTGCT and GACAGATCTCTT GCTGGA.
2.8. Flowcytometry
Splenic lymphocytes were treated with a lysis buffer containing 0.15 M ammonium chloride, 0.01 M potassium hydrogen carbonate and 0.1 mM EDTA at room temperature for 5 minutes to remove erythrocytes. After washing with RPMI medium containing 10% FCS, splenic lymphocytes (2 × 106) were stained with fluorescein-conjugated anti-TCR Vα2 mAb (clone B20.1; Becton Dickinson, San Jose, CA), and PE-Cy5-conjugated anti-mouse CD4 mAb (clone L3T4; Becton Dickinson) in the presence of 0.5% normal rat serum, and subsequently stained for dead cells with 7-amino-acitinomycin D (7-AAD, 2.5 μg/ml; Becton Dickinson). The stained lymphocytes were analyzed with a FACS Vantage Immunocytometry System (Becton Dickinson).
2.9. Blood Kinetics of OVA and PEG-OVA in Mice
Blood kinetics of OVA and PEG-OVA were measured using OVA labeled with fluorescein-5-maleimide at sulfhydryl groups on the molecule. An aliquot of 960 μl of 1.1 mM fluorescein-5-maleimide (Wako Pure Chemical Industries, Ltd.) in dimethylformamide was added to 36 ml of 5 mg/ml OVA in 0.1 M borate buffer (pH 7.5). The mixture was incubated under stirring at 37˚C for one hour, and dialyzed extensively against 0.1 M borate buffer (pH 7.5) to obtain fluorescein-labeled OVA (FOVA). A portion of F-OVA solution (18 ml of 5 mg/ml) was dialyzed against 0.1M sodium tetraborate buffer (pH 10.0), and subsequently reacted with 5.4 g of activated PEG2 to obtain PEG-conjugate of F-OVA (F-PEG-OVA) as described in Section 2.1. Both F-OVA and F-PEGOVA were purified extensively by ultrafiltration to remove residual fluorescein-maleimide. The protein concentrations of F-OVA and F-PEG-OVA were measured by the Biuret method [7], and both solutions were adjusted to 8 mg/ml of protein. F-OVA or F-PEG-OVA solution (200 μl) was injected into every 6 female C57BL/6 mice intraperitoneally. Blood samples were collected from each mouse at 15, 30, and 45 minutes, and 1, 2, 3, 8, 17.5, 45, 96 and 144 hours after injection. A 100 μl aliquot of each blood sample was immediately transferred to 900 μl of PBS containing 5 mM EDTA, and centrifuged to obtain the plasma sample. The concentrations of F-OVA and F-PEG-OVA in plasma samples were determined by fluorometric quantification using a Fuji FLA- 2000 imaging analyzer (Fujifilm Co., Ltd., Tokyo, Japan).
3. RESULTS
3.1. Immune Tolerance Induced with PEG-OVA Conjugates
PEG-OVA was prepared by reacting the free amino groups of the OVA molecule with activated PEG2 reagent having two methoxypolyethylene glycol chains with a molecular weight of 50 kilodaltons. The optimum degree of modification of the primary amino groups of the OVA molecule for tolerogenic conjugates was previously shown to be around 50% [12]. The degree of modification of the free amino groups of PEG-OVA used in the present study was 54%. To test the tolerogenicity of PEG-OVA conjugates, female C57BL/6 mice were pretreated with three weekly intraperitoneal injections of PEG-OVA, and subsequently immunized with OVA. The results are shown in Figure 1. Pre-treatment with PEGOVA induced a marked suppression of the IgG-class antiOVA immune response in mice, in keeping with the previous observations [7,8,12]. Similar profiles were observed for the anti-OVA responses of the IgM, IgG1, IgG2a, IgG2b and IgG3 subclasses (data not shown).
3.2. Response of Naïve OVA-Specific Th Cells towards Tolerogenic PEG-OVA
To characterize the response of OVA-specific naïve Th cells as elicited by tolerogenic PEG-OVA, splenic CD4+ T cells from OT-II mice with OVA-specific transgenic T-cell receptor genes [11] were labeled with carboxyfluorescein succinimidyl ester (CFSE), and were trans-
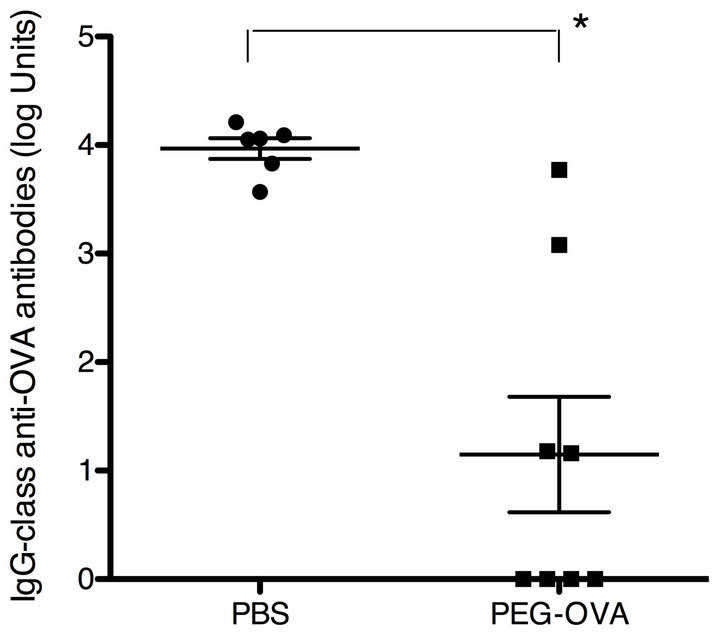
Figure 1. Immune tolerance induced by ovalbumin conjugated with polyethylene glycol (PEG-OVA). Female C57BL/6 mice received 3 weekly intraperitoneal injections of PEG-OVA (100 μg in 200 μl PBS) or PBS (200 μl) at weeks 0, 1 and 2. Subsequently, these mice were immunized with ovalbumin (250 μg in 200 μl PBS) at weeks 4, 5, 6 and 11. At week 13, blood samples were obtained from the orbital venous plexus using a capillary tube, and were allowed to clot at 4˚C. Sera were stored at −20˚C until analysis. Levels of IgG-class anti-OVA antibodies were measured by ELISA.
ferred into the histocompatible C57BL/6 mice. The recipient mice were subsequently injected intraperitoneally with either PBS, unconjugated OVA or tolerogenic PEGOVA. As shown in Figure 2, PEG-OVA, although tolerogenic with respect to the production of anti-OVA antibodies, induced a robust proliferation of naïve OVAspecific Th cells in vivo, as demonstrated by the appearance of CD4+ Th cells with gradually decreased fluorescence. The proliferative response induced by PEGOVA was comparable to or even higher than that induced by unconjugated OVA (Figures 2(d) and (e)).
3.3. Anergy of OVA-Specific Th Cells by Persistent Exposure to PEG-OVA
To determine the effect of longer exposure to tolerogenic PEG-OVA, female OT-II mice were injected intraperitoneally three times either with PBS, OVA or PEG-OVA at weeks 0, 1 and 2. Mice were sacrificed at week 3, and the splenic CD4+ Th cells were tested in vitro for their response towards varying concentrations of OVA323-339 peptide [17] that is recognized by OVAspecific Th cells. Figure 3 compares the dose-response curves observed for incorporation of BrdU in splenic Th cells from OT-II mice pretreated either with PBS, OVA or PEG-OVA. Half-maximal levels of the proliferation of naïve OT-II CD4+ T cells pretreated with PBS were observed at 1.6 × 10−9 M of OVA323-339 peptide. The cells
 (a)
(a) (b)
(b)
Figure 2. Proliferative response of OVA-specific Th cells from OT-II mice upon exposure to tolerogenic PEG-OVA. Splenic lymphocytes from OT-II mice were labeled with carboxyfluorescein succinimidyl ester (CFSE) as described in the MATERIALS AND METHODS section. Fluoresceinated cells (2 × 107) were resuspended in 200 μl of RPMI medium, and were transferred into the circulation of histocompatible C57BL/6 mice via the tail vein. The recipient mice were sub sequently injected intraperitoneally with either PBS, OVA or PEG-OVA as described in the MATERIALS AND METHODS section. Forty-eight hours after the injection, mice were sacrificed and the splenic lymphocytes were stained with phycoerythrine (PE)-labeled anti-CD4 mAb (L3T4). Two-color profiles are shown for spleen cells from recipient mice injected with PBS (a), OVA (b) and PEG-OVA (c). Numbers of OT-II CD4+ Th cells at 1 - 5 cell divisions were compared for two groups, one with three recipient mice injected with OVA (d) and the other with three recipient mice injected with PEG-OVA (e). A total of 105 spleen lymphocytes were analyzed for each recipient mouse.
from OT-II mice pretreated with OVA responded similarly, and the half-maximal level of response was observed at 2.0 × 10−9 M. In contrast, a higher concentration (1.1 × 10−8 M) of OVA323-339 peptide was required to observe the half-maximal response. The plateau level of the response observed for the cells from mice pretreated with PEG-OVA was also lower than those observed for the cells from mice pretreated with OVA or PBS.
3.4. Frequencies of Splenic OVA-Specific CD4+ Th Cells
The lower response observed in OT-II mice pretreated with PEG-OVA may have been partly due to the paucity of the OVA-specific CD4+ T cells in the spleen. Therefore, the frequency of TCR Vα2+CD4+ cells in the spleen was compared in OT-II mice pretreated with either PBSOVA or PEG-OVA. Representative two-color profiles are shown in Figures 4(a), (b) and (c). There was a significant reduction in the frequencies of splenic TCR Vα2+ CD4+ T cells in mice treated with tolerogenic PEG-OVA as compared with those from OT-II mice pretreated with PBS.
3.5. Cytokine Productions by OVA-Specific Th Cells Pretreated with Tolerogenic PEG-OVA
To characterize the pattern of cytokine productions of CD4+ Th cells exposed to tolerogenic PEG-OVA, OT-II mice were pretreated either with PBS, OVA or tolerogenic PEG-OVA as in Figure 3. Subsequently, splenic CD4+ T cells from these mice were cultured in the presence of 2.2 μM of OVA323-339 peptide in vitro. After 16 hours of incubation, the cells were harvested and analyzed for the expression of cytokine genes by real-time RT-PCR analysis. Figure 5 shows the relative abundance of transcripts as normalized on the basis of actin gene transcripts. Enhanced transcripts of IL-2, IL-4, IFN-γ, IL-10 and IL-17 were observed for the mice pretreated with OVA. In contrast, lower levels of cytokine gene transcripts were observed for the mice pretreated with PEG-OVA. The transcript of IL-2 was markedly suppressed by the pretreatment with PEG-OVA.
3.6. Blood Kinetics of OVA and PEG-OVA in Mice
The functional features of OVA-specific Th cells exposed to tolerogenic PEG-OVA were analogous to those observed for anergic Th cells, and presumably caused by the persistent exposure to the antigenic peptide of OVA323-339 derived from PEG-OVA. Therefore, in the present study, the blood kinetics of OVA and PEG-OVA were compared in mice. Figure 6 shows the blood kinetics of OVA and PEG-OVA. In mice injected with OVA, the concentration of OVA in the plasma reached a peak level (7 μg/ml) 30 minutes after the injection and then was cleared rapidly, becoming undetectable in 8 hours. In mice injected with PEG-OVA, the level reached a plateau (50 μg of OVA equivalent /ml) at 2 hours after the injection, and then declined very slowly. The estimated halflives for OVA and PEG-OVA were 2.0 and 59 hours, respectively. Therefore, PEG-OVA was found to be nearly 30 times as stable as OVA in the circulation, and was detectable in the plasma even 144 hours after the injecttion.
4. DISCUSSION
The mechanism of peripheral T-cell tolerance is thought to play a pivotal role in the regulation of the adaptive immune system, and has been a focus of extensive

Figure 3. Dose-response curves observed in vitro by various concentrations of antigenic OVA323-339 peptide of OVA-specific splenic CD4+ Th cells from OT-II mice pretreated with PBS (closed circles), with OVA (closed square) and with PEG-OVA (open triangles). The proliferative response was measured by the incorporation of bromodeoxyuridine (BrdU) as described in the MATERIALS AND METHODS section. Half-maximal levels of the responses were observed at 1.6 × 10−9 M of the peptide concentration for mice pretreated with PBS, at 2.0 × 10−9 M for mice pretreated with OVA, and at 11 × 10−9 M for mice pretreated with PEG-OVA.
studies aiming to develop an effective method of specific intervention in immune-mediated disorders. In classical studies, the routes of administration and dosage regimen of exogenous proteins that optimize the T-cell tolerance in the experimental animals have been explored [27]. Recently acquired information on the cellular and molecular basis of antigen recognition by T cells has led to the proposal of several strategies for the specific downregulation of pathogenic T cells. For example, altered peptide ligands (antigenic peptides with amino acid substitutions at TCR contact sites) were clinically tested for their ability to antagonize pathogenic T cells in patients with multiple sclerosis [28]. Preferential use of a particular TCR Vβ (Vβ 8) gene element observed in the pathogenic T cells in the murine model of experimental allergic encephalomyelitis (EAE) allowed its successful treatment with Vβ 8-specific monoclonal antibodies [29]. Genetically engineered tolerogenic dendritic cells were tested for their ability to suppress the graft rejection [30]. To date, however, none of these approaches has proven to be practical.
The technique of covalent attachment of polyethylene glycol has been studied for nearly 40 years, and was successfully applied to improve the efficacy of therapeutic enzymes and cytokines [3-6]. However, the antigenspecific immune tolerance induced by PEG-protein antigen conjugates, which was originally described by Lee and Sehon [7-9], has not been fully worked out. In the present study, we examined the response and fate of anti-
 (a)
(a) (b)
(b)
Figure 4. Reduction in the frequency of splenic OVA-specific CD4+ Th cells from OT-II mice treated with tolerogenic PEG-OVA. Female OT-II mice were pretreated either with PBS, OVA or PEG-OVA as described in the MATERIALS AND METHODS section. One week after the last treatment, mice were sacrificed and analyzed for the frequencies of splenic TCR Vα2+CD4+ T cells. Representative profiles are shown for an OT-II mouse treated with PBS (a), OVA (b) and PEGOVA (c). Numbers in quadrants are the frequencies (%) in lymphocytes. (d) Average frequencies of Vα2+CD4+ cells in the splenic CD4+ Th cells.
gen-specific Th cells upon encountering tolerogenic PEGprotein antigen conjugate. The availability of transgenic mice expressing the OVA-specific TCR gene [11] has enabled studies using the same model antigen as used in the classical studies.
In keeping with the results of previous studies [7,12], pretreatment of C57BL/6 mice with our PEG-OVA preparation induced the OVA-specific immune tolerance. The suppressive effect of PEG-OVA on the OVA-specific antibody production was not restricted to particular immunoglobulin isotypes (data not shown). Therefore, skewed differentiation into particular Th subsets was not likely involved. PEG-OVA was recognized by CFSE-labeled OVA-specific naïve Th cells and induced their robust proliferation. However, treatment of OT-II mice with PEG-OVA resulted in an anergic state of OVA-specific Th cells that was characterized by a low response rate and a markedly reduced ability to produce IL-2 and other cytokines, upon stimulation in vitro with antigenic peptide OVA323-339. There was also a slight but significant reduction of the frequencies of the clono-
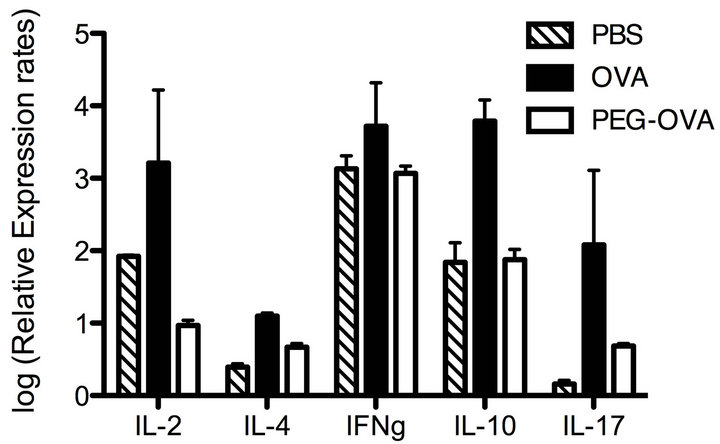
Figure 5. Cytokine gene expressions as induced by the stimulation with antigenic OVA323-339 peptide in vitro in splenic CD4+ Th cells from OT-II mice pretreated with PBS, OVA and PEG-OVA. Three groups of OT-II mice were pretreated with PBS, OVA or PEG-OVA as in Figure 1. One week after the last treatment, mice were sacrificed and spleen cells (4 × 107 cells) were cultured in vitro in the presence of 2.2 μM of OVA323-339 peptide in RPMI medium supplemented with 10% FCS and 50 μM of 2-mercaptoethanol. After 16 hours, the cells were harvested and lysed with Trisol Reagent. Cytoplasmic RNAs were reverse-transcribed by reverse transcriptase, and the relative abundance of cytokine gene transcripts was measured with reference to the actin-gene transcript levels by real-time PCR analysis as described in the MATERIALS AND METHODS section.
typic TCR Vα2+CD4+ Th cells in the spleens of OT-II mice treated with PEG-OVA.
In the process of tolerance induction, the functions of antigen-specific Th cells are theoretically downregulated in the periphery by three alternative mechanisms. The first is clonal deletion of antigen-specific Th cells by apoptosis [31]. The second is the establishment of clonal anergy that is intrinsic to the specific Th cells [32]. The third is clonal suppression by regulatory T (Treg) cells [33]. The present and previous data suggest that clonal anergy was the most common fate of OVA-specific naïve Th cells upon persistent exposure to PEG-OVA. As to the possibility of clonal deletion of Th cell precursors in the thymus, we could not observe any marked distortion of TCR Vα2+ thymocyte subsets as dissected by CD4 and CD8 expressions in the thymus of mice treated with PEG-OVA (data not shown). Nevertheless, there was a slight reduction in the frequencies of clonotypic TCR Vα2+CD4+ T cells in the spleens of OT-II mice pretreated with PEG-OVA. Therefore, it is possible that clonal deletion of OVA-specific Th cells may have partly contributed to the observed OVA-specific unresponsiveness. In early studies, carrier-specific Th cell tolerance induced with PEG-OVA was explained by the involvement of specific suppressor T (Ts) cells that were thought to be responsible for the downregulation of Th cells [34,35]. As to the potential involvement of regulatory T (Treg) cells, we could not detect any marked increase of the

Figure 6. Blood kinetics in mice treated with OVA (closed circles) and PEG-OVA (open circles). Sulfhydryl groups of the OVA molecule were labeled with fluorescein using fluorescein maleimide. Fluorescein-labeled OVA was subsequently conjugated with activated PEG2 reagent as described in the MATERIALS AND METHODS section. Two groups of six C57BL/6 mice each received intraperitoneal injection of either fluorescein-labeled OVA or PEG-conjugated fluorescein-labeled OVA. Blood samples were collected at various time periods, and the time course of changes in plasma fluorescence levels was measured by using an FLA-2000 fluorescence analyzer (Fujifilm, Tokyo, Japan).
frequencies of Foxp3+ cells either in the TCR Vα2+CD4+ population or in the TCR Vα2−CD4+ population in the spleen (data not shown).
The initial robust response, and the subsequent anergy of naïve OT-II Th cells upon persistent exposure to tolerogenic PEG-OVA observed in the present studies, were quite analogous to those reported for the OVA-specific transgenic Th cells transferred into the histocompatible mice with systemic expression of the transgenic OVA gene. Barron, et al. [36] reported that naïve OVAspecific Th cells from DO11.10 mice transferred into histocompatible BALB/c mice with systemic expression of transgenic OVA proliferated vigorously in the periphery. Nevertheless, these cells eventually ceased to prolixferate and became anergic, exhibiting reduced production of IL-2, IL-4 or IFN-γ. They observed that some of the OVA-specific Th cells were lost by apoptosis upon the sustained exposure to OVA. Genetic manipulation of OVA-specific Th cells to inhibit their apoptosis did not prevent their anergy upon persistent exposure to transgenic OVA. They concluded that induction of clonal anergy is the major mechanism of self-tolerance of Th cells in the periphery.
Because the above-described experiments using mice with systemic expression of a transgenic OVA gene are generally accepted as a model of peripheral tolerance of autoreactive Th cells, the Th cell tolerance induced with PEG-OVA may be concluded to occur via a mechanism that is normally operative for the establishment of selftolerance. This type of tolerogenic capacity of PEG-OVA likely arises from the enhanced plasma half-life of PEGOVA in the circulation. Highly enhanced stability has often been reported for the PEG-conjugates of enzymes, and is generally the basis of their improved therapeutic activities [3-6]. In the present study, we compared the blood kinetics of OVA and PEG-OVA, and observed that stability of OVA in the circulation was indeed greatly enhanced by conjugation with PEG. Persistent presentation of the OVA323-339 peptide originated from PEG-OVA, and presumably the absence of costimulatory signals for effective Th cell differentiation might be the bases of the clonal anergy of Th cells as induced with PEG-OVA.
PEG-protein antigen conjugates demand attention as potential tools for specific immune intervention. Better understanding of the nature of their tolerogenic antigen presentation could lead to their application as tools for specific immune interventions.
5. CONCLUSION
Tolerogenic polyethylene glycol (PEG) conjugate of ovalbumin (OVA) was recognized by transgenic OVAspecific Th cells, and induced their robust proliferation. However, upon prolonged exposure to PEG-OVA, OVAspecific Th cells became anergic. This process was analogous to that observed for the peripheral tolerance of autoreactive Th cells. The tolerogenic potential of PEGOVA is likely due to the highly enhanced stability in the circulation, and the inability to elicit costimulatory signals for an effective immune response.
6. ACKNOWLEDGEMENTS
We thank Noriko Iida for technical assistance. This work was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Science, Technology, Sports and Culture, Japan.