1. Introduction
Livestock are domesticated animals raised in agricultural setting to produce commodities such as food, fiber and labour [1] . In Nigeria, meat derived from bovine (cattle) provides major animal protein for the people. Animal proteins are used to build new protoplasm, used for growth and repair of tissues. Animal proteins also have a higher concentration of sulphur containing amino acids that get metabolized to acid-generating metabolites [2] .
Fascioliasis is a major public health problem in many areas of the world especially in developing countries where there is poor sanitation, poor personal hygiene, poverty and poor animal husbandry [3] . Millions of carcasses and livers are lost due to damages caused by Fasciola infection in animals such as cattle, sheep and goats [1] . It is caused by trematodes belonging to the genus Fasciola commonly known as Liver fluke. Fascioliasis is a highly pathogenic disease of clinical and veterinary importance [4] . Incidence of this disease has been reported in many countries including Nigeria, Pakistan, China, United States of America and Iran [3] [4] .
Ruminant hosts become infected when forage with metacercarial cyst is ingested. They can also be infected when ingesting cysts are suspended in soil and detritus while drinking water. Ingested parasite finds its way to intra-hepatic biliary duct or hepatic parenchyma and later to the bile duct where it resides [5] .
Infected ruminant liver usually experiences traumatic injury, giving rise to diffusely hepatic parenchyma containing haemorrhagic streaks or foci. The animal may experience weight loss, anaemia and general depression. The liver may be enlarged and show abnormal functions, and blood leukocytosis with eosinophilia in response to Cathepsin B antigen secreted by juvenile fluke may be observed [6] . Complicated expression due to synergy with Clostridium noryi and Clostridium haemolyticum results in black diseases referred to as infectious necrotis hepatitis. This infection makes the liver appear black in colour [5] .
Urbanization, migration and developmental practices, such as dam building and irrigation, have increased the population at risk and incidence of human infection has increased significantly over the past 20 years [7] . Hence, evaluating cattle from slaughtered houses records may prove useful in assessing the potential risk they pose to human [8] . This study is aimed at evaluating the prevalence of bovine fascioliasis in slaughter house within Anyigba, Dekina Local government, Kogi State.
2. Materials and Methods
2.1. Study Area
The study was conducted at Anyigba, the largest town in Dekina Local Government Area, Kogi State, Nigeria. Anyigba is located on Latitude 7˚15' - 7˚29'N and Longitude 7˚11' - 7˚32'E, with a total area of 11.07506 sqkm. Anyigba has a main abattoir where several cattle are slaughtered daily.
2.2. Sample Collection
Daily visits were paid on rotational basis to the slaughtered house for the purpose of collecting bile samples from slaughtered cattle between 6 a.m. and 8 a.m. each sampling day. Samples were collected over a period of 4 weeks. Intact gall bladder removed from randomly selected cattle slaughtered at the abattoirs was collected from butchers and the openings tied with rubber bands. They were placed in properly labeled small plastic bowls with lids and preserved with few drops of 10% formalin. During bile collection, the sex of the respective animals whose bile were collected was determined by examination of their external genitalia. The samples were transported to the Laboratory of the Department of Biological Sciences, Faculty of Natural Sciences, Kogi State University, for the determination of prevalence and intensity of Fasciola hepatica infections.
2.3. Detection, Identification and Counting of Adult Fasciola
Gall bladder examination was carried out as described by Schillhorn et al. [9] . The bile was emptied into separate glass beakers from the gall bladders of the cattle and the goat by incision made with dissecting scissors. The volume of the respective bile emptied was measured and recorded. With the aid of forceps and magnifying lens, adult Fasciola specimens present in the gall bladders of naturally infected cattle and goats were picked, counted and recorded. Identification of adult Fasciola specimens was aided by morphological keys by Soulsby [10] and WHO [11] .
2.4. Microscopic Examination of Bile
Bile samples collected from each gall bladder was mixed thoroughly by shaking for random distribution of eggs. 10 ml of each sample was introduced into a labelled centrifuge tube and centrifuged at 1500 rpm for ten minutes; in a centrifuge machine. Few ml of water was added to the sediments to make it measure up to 1 ml. This is for the purpose of having a uniform volume for the bile samples and also to make it easily observable under the microscope. Using a micro-pipette, 0.1 ml of each sample was introduced on a slide covered with cover slip and examined under ×40 magnification of a research microscope for the presence of Fasciola hepatica eggs. Identification of Fasciola eggs was based on pictorial keys by Soulsby [12] . The total number of Fasciola eggs were determined by calculating the dilution factor and recorded. Therefore for each sample, number of eggs found is multiplied by 10 which is the dilution factor to get actual number of eggs per bile sample.
2.5. Statistical Analysis
Data obtained was analyzed using IBM SPSS software version 21.0 for windows. Descriptive statistics was used in the calculation of prevalence. Chi-square test was used to test the level of significance in parasite prevalence and intensities between bovine age and sex. Significance was determined at (P > 0.05) that is 95% confidence interval.
3. Results
Out of the 300 bile samples of bovine (cattle) examined for the presence of F. hepatica and D. dendriticum eggs, 33.0% (99 cattle) were positive for F. hepatica while 39.0% (117 cattle) were positive for D. dendriticum.
Sex specific prevalence of F. hepatica revealed that the males were more infected than the females with the males and females having a prevalence of 37.4% (58 cattle) and 28.3% (41 cattle) respectively. For D. dendriticum, also the males were more infected than the females with a prevalence of 41.9% (65 cattle) in male and 35.9% (52 cattle) in female (Table 1). No significant difference (P > 0.05) was observed in the prevalence between the sexes in both F. hepatica and D. dendriticum.
Age specific prevalence of F. hepatica revealed that age of 0 - 2 years were more infected than age of 2 - 4 years with prevalence of 18.7% (29 cattle) and 48.3% (70 cattle) respectively (Table 1). For D. dendriticum, age of 2 - 4 years were more infected than age of 0 - 2 years with a prevalence of 59.3% (86 cattle) and 20.0% (31 cattle) respectively. Significant difference (P < 0.05) was observed
![]()
Table 1. Prevalence of Fasciola hepatica and Dicrocoelium dendtriticum in Slaughter Houses within Anyigba, Dekina Local Government, Kogi State.
ns―not significant at P > 0.05; *―significant at P < 0.05.
in the prevalence between the age groups in both F. hepatica and D. dendriticum with the adult cattle having more infections.
Out of the 300 bile samples of bovine (cattle) examined, 22.3% (67 cattle) were co-infected with F. hepatica and D. dendriticum. Males were more co-infected than females having a prevalence of 24.5% (38 cattle) and 20.0% (29 cattle) respectively. Based on the age, the age of 2 - 4 years were more co-infected with these parasites than age of 0 - 2 years with a prevalence of 32.4% (47 cattle) and 12.9% (20 cattle) respectively (Table 2). Statistically, significant difference (P < 0.05) in co-infection was observed between the adult and young cattle.
4. Discussion
Fasciola hepatica and Dicrocoelium dendriticum were identified from the bile of cattle slaughtered at abattoir in Anyigba, Kogi State (Plate 1). The presence of these two parasites in cattle had earlier been reported by Talukder et al. [13] , Ozung et al. [14] and Shaikh et al. [15] . The animal exhibited no visible signs and symptoms except that some of them were pale and emaciated. This observation is in line with Tolan [16] , who reported that about 50% of fascioliasis is asymptomatic.
A total of 33.0% of the cattle were infected with F. hepatica while 39.0% was infected with D. dendriticum. A prevalence of 33.0% of F. hepatica among cattle reported in this work differ significantly from 14.8% prevalence reported by
![]()
Table 2. Co-infection of F. hepatica and D. dendriticum in cattle slaughtered at abattoir in Anyigba, Dekina Local Government, Kogi State.
ns―not significant at P > 0.05; *―significant at P < 0.05.
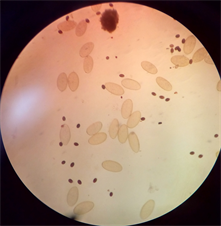 (a)
(a)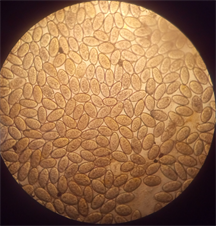 (b)
(b)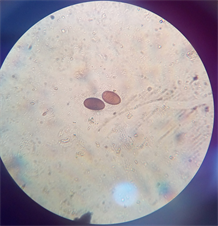 (c)
(c)
Plate 1. (a) Dicrocoelium dendriticum; (b) Fasciola hepatica; (c) Mixed infection of F. hepatica and D. dendriticum.
Shaikh et al. [15] in Pakistan and 50.52% reported by Ozung et al. [14] in Ikom (Nigeria). There were single infection of F. hepatica and D. dendriticum. There were also mixed infections of F. hepatica and D. dendriticum. The prevalence of F. hepatica obtained in this study was higher than 23.3% prevalence reported by Njoku [17] from Imo Abattoirs, Nigeria. The difference in prevalence may be due to difference in resistance to infection because of the host breed and grazing habits [18] . Also, difference in infection may have been influenced by the varying ecological and climatic conditions of the area where they might have grazed upon before getting to the abattoir. This agrees with the reports of Afrakhosrayi [6] and Keiser and Utzinger [19] .
The observed high prevalence of single infection with F. hepatica, 33.0% in bovine conforms to the high prevalence of 50.52% reported by Ozung [14] in Ikom (Nigeria). This may be due to the nature of slaughtering location, availability of forage inhabited by snail intermediate host and exposure of the animals to the forage [15] .
The FAO [20] reported a range of 17% - 56% prevalence of D. dentriticum in Nigeria. Single infection with D. dentriticum in bovine reported in this study was 39.0% which conforms with 30.16% reported by Hassan and Anwo [21] from Isheri Olofin abattoir in Ogun-State, Nigeria.
The higher D. dentriticum prevalence is noteworthy considering reports that dicrocoeliasis is poorly known, often underestimated by researchers and practitioners. In many countries availability of few effective drugs, difficulty of diagnosis and presence of poly-parasitic infections which mask the pathology of the disease are also part of the problem [20] . This trend may constitute a significant animal health issue of emerging disease. The prevalence of both single and mixed parasitic infections was high in bovine, this owns to the fact that most of the cattle brought to the slaughter houses were extensively raised. So, the rate of the bovine ingesting the metacecariae from vegetation could be high. It was observed that infection rate was high in both male and female bovine with 37.4% (58) and 28.3% (41) for single parasite infection while 24.5% (38) and 20.0% (29) for mixed infections, the animals must have grazed on contaminated areas and exposure of the animals to the forage while travelling through their trade route [15] . Prevalence of bovine Fasciola and Dicrocoelium infections was high in cattle examined in Anyigba abattoir (P < 0.05). This could be as a result of the area having an exposed and contaminated water bodies with aquatic vegetation surrounding them. These vegetation support the growth of water snail that carries the parasite [22] . Also, acquiring infection by cattle is not limited to the source, cattle are raised long enough at slaughtering location and this could influence prevalence patterns.
Although in this study, liver samples were not analyzed directly, it is expected that the result obtained using gall bladder contents is an indication of liver infections. Cawdery et al. [23] had suggested that a prevalence of up to 25% is an indication of the level of infection in which most of the animals affected would have had their liver damaged, rendering them unfit for human consumption. The prevalence of 33.0% for F. hepatica placed a probability that each liver supplied to the market is unfit for public consumption at 50%. This is consistent with the general observation that most of the cattle brought to the abattoir for slaughter were apparently diseased and weak. Some barely made to the abattoir alive. It was also observed that some of the gall bladder were enlarged resulting to the high volume of the bile content. This also is an indication of infected liver owning to the assertions made by the butchers at the abattoir. This is true to a large extent; since an infected liver is often enlarged, so also the gall bladder which is closely linked to it will be enlarged as a result of the obstruction of the bile ducts caused by these flukes which led to accumulation of the bile fluid.
5. Conclusion
The result of this study clearly indicates that prevalence of liver fluke infections remains high in the study area. The overall prevalence of trematodes parasites eggs in gall-bladder observed in cattle (33.0%) was significantly high. The findings of this present study imply that in spite of major concerns about economic losses and large volume of documentation on animal health implications of helminthes infections, efforts to alleviate problems of animal health and productivity are yet to make any significant impact and this poses threat on human. Investigation on the pattern of infections in cattle slaughtered should be encouraged.