Synthesis and Anti-Tumor Activities of Fluoride-Containing Gossypol Derivatives Compounds ()
1. Introduction
Gossypol, the polyphenolic constituent isolated from cottonseeds, has been used as a male antifertility drug for a long time, and has been demonstrated to exhibit excellent anti-tumor activity towards multiple cancer types [ 1 ]. Up to now, gossypol has been showed to exhibit anti-tumor activities towards a wide range of tumors, such as Ehrlich ascites tumor cells [ 2 ], SW-13 adrenocortical carcinoma cells [ 3 ], hormone-dependent and hormone-independent breast carcinoma [ 4 , 5 ], colon carcinoma cell line HT-29 and LoVo [ 6 ], human pancreatic cancer cell line [ 7 ], prostate cancer cell lines [ 8 ], head and neck cancer cells [ 9 , 10 ]. In addition, many synthesized gossypol derivatives and analogues possess disease-inhibiting activities [ 11 ], as anti-parasitic [ 12 , 13 ], anti-malarial [ 14 - 16 ], anti-HIV [ 17 - 19 ] and anticancer [ 20 - 23 ]. Derivatives such as gossypol Schiff bases prepared by modifying gossypol’s aldehyde groups were supposed to reduce its host toxicity (Figure 1) while retaining or enhancing its therapeutic effects [ 24 ].
Fluorine substituents have become a widespread and important drug component. Organofluorine affects nearly all physical and adsorption, distribution, metabolism, and excretion properties of a lead compound. Its inductive effects are relatively well understood and enhancing bioavailability [ 25 - 27 ]. Top-selling fluorinated pharmaceuticals include the antidepressant fluoxetine (Prozac), the cholesterol-lowering drug atorvastatin (Lipitor), and the antibacterial ciprofloxacin (Ciprobay) (Figure 2) [ 28 ].
The aforementioned findings stimulated our interest in designing and synthesizing a series of fluoride-containing gossypol Schiff base derivatives (Figure 3) which were anticipated to be a much higher anti-tumor activity yet lower systemic toxicity than gossypol. The activity of the target compounds were evaluated by three human cancer cell lines (HeLa, A-549 and BGC-823) and a normal cell line (VEC) in vitro by using MTT cell proliferation assays. To the best of our knowledge, the biological evaluation of fluoride-containing gossypol derivatives for in vitro anti-tumor activity is not reported [ 11 ].
![]()
Figure 1. Chemical structure of gossypol (The highlighted aldehyde groups were supposed to be toxicity).
![]()
Figure 3. Designing of fluoride-containing gossypol derivatives.
2. Results and Discussion
2.1. Synthesis of Gossypol Derivatives
Fluoride-containing gossypol derivatives 3a-3f were prepared by the condensation reaction of gossypol1 with fluoride-containing aromatic amine 2a-2f (Scheme 1).
Reaction conditions: For preparation of fluoride-containing derivatives 3. The gossypol (0.001 mol) dissolved in an excess of methanol (40 ml) was mixed with 0.004 mol suitable compound as shown in Table 1 and the stirrer was added later. Next, put the reactor into the heat collection type constant temperature heating magnetic stirrer with 65˚C, the mixture was heated and refluxed for 4 hours to precipitate the yellow solid which was recovered by filtration and washed with petroleum ether-ethyl acetate (16:1). Then, the precipitate was purified by recrystallization from petroleum ether-ethyl acetate; For Compound 3, the structure can be interpreted by 1H NMR (Figure 4) [ 22 ].
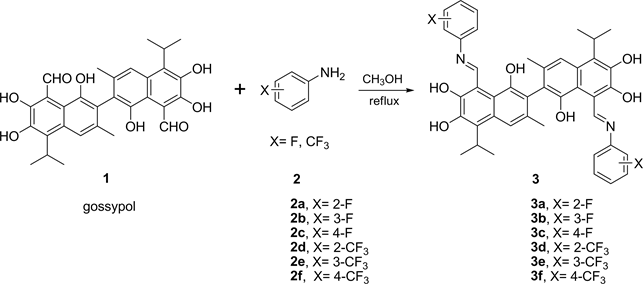
Scheme 1. Synthesis of fluoride-containing gossypol derivatives 3a-3f.
![]()
Figure 4. Attribution of proton NMR signals.
![]()
Table 1. The suitable compounds required for the preparation of fluoro-gossypol derivatives.
8,8'-bis((E)-(2-fluorophenylimino)methyl)-5,5'-diisopropyl-3,3'-dimethyl-2,2'-binaphthyl-1,1',6,6',7,7'-hexaol (3a): yellow solid, Yield: 0.61 g, yield: 75%; mp, 255˚C - 257˚C; IR (KBr, cm−1): 3352, 3102, 1580, 1231, cm−1; 1H NMR (400 MHz, CDCl3): δ = 15.25 (d, J = 11.2 Hz, 2H, H-f), 10.28 (d, J = 11.2 Hz, 2H, H-h), 8.58 (s, 2H, H-b), 8.28 (s, 2H, H-e), 7.54 (s, 2H, H-g), 7.30 - 7.60 (m, 8H, H-, j, k, l, m), 3.81 (m, 2H, H-d), 2.05 (s, 6H, H-a), 1.52 (d, 12H, H-c); 13C NMR (100 MHz, CDCl3): δ = 173.1, 160.8, 158.5, 153.7, 150.3, 146.1, 136.3, 132.5, 128.9, 127.9, 121.1, 120.3, 119.8, 119.8, 119.1, 117.1, 116.9, 116.7, 115.4, 105.7, 26.3, 20.1, 20.2 ppm; HRMS EI (m/z): calcd for C42H38F2N2O6, 704.2695; found, 704.2698.
8,8'-bis((E)-(3-fluorophenylimino)methyl)-5,5'-diisopropyl-3,3'-dimethyl-2,2'-binaphthyl-1,1',6,6',7,7'-hexaol (3b): yellow solid, Yield: 0.58 g, yield: 71%; mp, 263˚C - 265˚C; IR (KBr, cm−1): 3357, 3100, 1580, 1223, cm−1; 1H NMR (400 MHz, CDCl3): δ = 15.12 (d, J = 12.0 Hz, 2H, H-f), 10.20 (d, J = 12.0 Hz, 2H, H-h), 8.57 (s, 2H, H-b), 8.27 (s, 2H, H-e), 7.56 (s, 2H, H-g), 7.31 - 7.62 (m, 8H, H-i, k, l, m), 3.80 (m, 2H, H-d), 2.05 (s, 6H, H-a), 1.52 (d, 12H, H-c); 13C NMR (100 MHz, CDCl3): δ = 173.0, 160.8, 158.5, 153.6, 150.1, 146.2, 136.3, 132.5, 128.9, 127.9, 121.3, 120.3, 119.1, 119.0, 118.9, 117.1, 116.9, 116.2, 115.2, 105.6, 26.2, 20.1, 20.1 ppm; HRMS EI (m/z): calcd for C42H38F2N2O6, 704.2695; found, 704.2698.
8,8'-bis((E)-(4-fluorophenylimino)methyl)-5,5'-diisopropyl-3,3'-dimethyl-2,2'-binaphthyl-1,1',6,6',7,7'-hexaol (3c): yellow solid, Yield: 0.69 g, yield: 85%; mp, 265˚C - 267˚C; IR (KBr, cm−1): 3351, 3188, 1580, 1210, cm−1; 1H NMR (400 MHz, CDCl3): δ = 14.94 (d, J = 11.6 Hz, 2H, H-f), 10.31 (d, J = 11.6 Hz, 2H, H-h), 8.54 (s, 2H, H-b), 8.24 (s, 2H, H-e), 7.52 (s, 2H, H-g), 7.29 - 7.57 (m, 8H, H-i, j, l, m), 3.80 (m, 2H, H-d), 2.04 (s, 6H, H-a), 1.51 (d, 12H, H-c); 13C NMR (100 MHz,CDCl3):δ = 173.6, 160.9, 158.5, 153.9, 150.1, 146.1, 136.3, 132.5, 128.9, 127.8, 121.1, 119.8, 119.8, 117.1, 116.9, 116.7, 115.4, 105.6, 26.6, 20.2, 20.2 ppm; HRMS EI (m/z): calcd for C42H38F2N2O6, 704.2695; found, 704.2698.
5,5'-diisopropyl-3,3'-dimethyl-8,8'-bis((E)-(2-(trifluoromethyl) phenylimino)methyl)-2,2'-binaphthyl- 1,1',6,6',7,7'-hexaol (3d): yellow solid, Yield: 0.53 g, yield: 64%; mp, 251˚C - 253˚C; IR (KBr, cm−1): 3360, 3120, 1620, 1330, cm−1; 1H NMR (400 MHz, CDCl3): δ = 15.21 (d, J = 11.0 Hz, 2H, H-f), 10.10 (d, J = 11.0 Hz, 2H, H-h), 7.74 (s, 2H, H-b), 7.62 (s, 2H, H-e), 7.68 - 7.26 (m, 8H, H-j, k, l, m), 5.77 (s, 2H, H-g), 3.76 - 3.66 (m, 2H, H-d), 2.15 (s, 6H, H-a), 1.57 - 1.51 (m, 12H, H-c); 13C NMR (100 MHz, CDCl3), δ 175.0, 154.8, 149.5, 146.8, 138.6, 133.5, 133.3, 130.2, 129.5, 127.1, 127.1, 127.0, 126.9, 125.3, 125.0, 122.3, 121.2, 120.9, 119.0, 118.9, 116.4, 114.2, 106.4, 27.6, 20.2, 20.2, 20.1 ppm; HRMS EI (m/z): calcd for C44H38F6N2O6, 804.2630; found, 804.2634.
5,5'-diisopropyl-3,3'-dimethyl-8,8'-bis((E)-(3-(trifluoromethyl)phenylimino)methyl)-2, 2'-binaphthyl- 1,1',6,6',7,7'-hexaol (3e): yellow solid, Yield: 0.68 g, yield: n83%; mp, 262˚C - 264˚C; IR (KBr, cm−1): 3360, 3120, 1620, 1328, cm−1; 1H NMR (400 MHz, CDCl3): δ = 15.02 (d, J = 11.7 Hz, 2H, H-f), 10.14 (d, J = 11.7 Hz, 2H, H-h), 7.79 (s, 2H, H-b), 7.65 (s, 2H, H-e), 7.54−7.41 (m, 8H, H-i, k, l, m), 5.79 (s, 2H, H-g), 3.77 - 3.69 (m, 2H, H-d), 2.16 (s, 6H, H-a), 1.58 - 1.53 (m,12H, H-c); 13C NMR (100 MHz, CDCl3), δ 175.2, 153.5, 149.5, 146.9, 140.2, 133.3, 133.1, 132.7, 132.3, 132.0, 130.5, 130.1, 129.6, 124.9, 122.2, 122.1, 122.1, 121.2, 119.0, 116.4, 114.9, 114.9, 114.1, 105.7, 27.6, 20.3, 20.2, 20.1 ppm; HRMS EI (m/z): calcd for C44H38F6N2O6, 804.2630; found, 804.2634.
5,5'-diisopropyl-3,3'-dimethyl-8,8'-bis((E)-(4-(trifluoromethyl)phenylimino)methyl)-2,2'-binaphthyl-1,1',6,6',7,7'-hexaol (3f): yellow solid, Yield: 0.51 g, yield: 62%; mp, 261˚C - 263˚C; IR (KBr, cm−1): 3365, 3129, 1622, 1335, cm−1; 1H NMR (400 MHz, CDCl3): δ = 14.89 (d, J = 12.0 Hz, 2H, H-f), 10.15 (d, J = 12.0 Hz, 2H, H-h), 7.77 (s, 2H, H-b), 7.65 - 7.55 (m, 6H, H-e, i, j, l, m), 7.37 (d, J = 8.4 Hz, 4H), 5.75 (s, 2H, H-g), 3.78 - 3.68 (m, 2H, H-d), 2.16 (s, 6H, H-a), 1.58 - 1.55 (m, 12H, H-c); 13C NMR (100 MHz, CDCl3), 13C NMR (100 MHz,CDCl3): δ = 175.5, 153.1, 149.8, 149.6, 146.9, 142.4, 140.9, 133.4, 130.2, 129.7, 127.2, 127.1, 127.1, 119.0, 117.9, 116.5, 105.8, 105.8, 27.6, 20.3, 20.2, 20.1; HRMS EI (m/z): calcd for C44H38F6N2O6, 804.2630; found, 804.2634.
2.2. Anti-Tumor Activities
All the fluoride-containing gossypol derivatives were screened for in vitro cytotoxicity against three human cancer cell lines (HeLa, A-549 and BGC-823) and a normal cell line (VEC) by MTT assay. In vitro, the cytotoxic activities of gossypol and fluoride-containing gossypol Schiff base derivatives were determined by the MTT cytotoxicity assay, which was performed in 96-well plates. The tumor cell line panel consisted of HeLa (human cervical carcinoma), A-549 (human lung carcinoma), BGC-823 (human gastric carcinoma), VEC (human vascular endothelial cells) (final concentration in the growth medium was (2 - 4) × 104/mL). MTT solution (20 μL/well) was added after cells were treated with drug for 48 h, and cells were incubated for a further 4 h at 37˚C. The purple form azan crystals were dissolved in 150 μL DMSO. After 5 min, the plates were read on an automated micro plate spectrophotometer at 570 nm. Assays were performed in triplicate in three independent experiments. The concentration required for 50% inhibition of cell viability (IC50) what was calculated. In all of these experiments, three replicate wells were used to determine each point.
As shown in Table 2, it was found that substituent changes on the gossypol’s aldehyde groups have a great influence on the normal cells activity (3a - 3f), which exhibited lessened cytotoxicity against normal cells (VEC). The data reveal that compounds 3a, 3c and 3f exhibited superior anticancer activity against HeLa, compounds 3b, 3c, 3e and 3f exhibited superior anticancer activity against A-549, compounds 3b, 3c and 3f exhibited superior anticancer activity against BGC-823 compared to gossypol. In particular, fluorine substituent at the para positions of the phenyl ring showed remarkable inhibitory effects on HeLa (3c: IC50 = 14.2 μM, 3f: IC50 = 8.34 μM), A-549 (3c: IC50 = 6.32 μM, 3f: IC50 = 9.76 μM) and BGC-823 cells (3c: IC50 = 8.62 μM, 3f: IC50 = 4.36 μM), which represented superior antitumor activity compared to gossypol (IC50 = 23.3 μM against HeLa, IC50 = 19.1 μM against A-549, IC50 = 17.1 μM against BGC-823,), respectively. Moreover, fluoride-containing gossypol derivatives 3c and 3f exhibit good safety profiles (IC50 > 100 μM against VEC).
3. Conclusion
In summary, a series of novel fluoride-containing gossypol Schiff base derivatives were synthesized and tested for their in vitro cytotoxic activities against three human cancer cell lines(HeLa, A-549 and BGC-823) and a normal cell line (VEC) by using MTT cell proliferation assays. All the compounds exhibited lessened cytotoxicity against normal cells (VEC). Results revealed that compounds 3a, 3c and 3f exhibited superior anticancer activity against HeLa, compounds 3b, 3c, 3e and 3f exhibited superior anticancer activity against A-549, compounds 3b, 3c and 3f exhibited superior anticancer activity against BGC-823 compared to gossypol. In particular, fluorine substituent at the para positions of the phenyl ring showed remarkable inhibitory effects on HeLa (3c: IC50 = 14.2 μM, 3f: IC50 = 8.34 μM), A-549 (3c: IC50 = 6.32 μM, 3f: IC50 = 9.76 μM) and BGC-823 cells (3c: IC50 = 8.62 μM, 3f: IC50 = 4.36 μM). Moreover, fluoride-containing gossypol derivatives 3c and 3f exhibit good safety profiles (IC50 > 100 μM against VEC). And thus as anti-cancer drug, fluoride-containing gossypol Schiff base derivatives has a better application prospects. Studies to determine the in vivo pharmacokinetics and efficacy of compounds 3c and 3f against some selected tumor cell lines are currently underway.
![]()
Table 2. The inhibiting effect of compounds 3a - 3f to HeLa, A-549 and BGC-823 cell lines in vitro.
aIC50 values were the means of three independent experiments run in duplicate.
Acknowledgements
The authors thank the High-level Talent Introduction Foundation of Southern Medical University (C1033520), Natural Science Foundation of China (81102823), Guangdong Natural Science Foundation (2014A030310342) and the Science and Technology Program of Guangdong Province (2015A010105015) for financial support.