1. Introduction
Viniculture is a very important activity in Santa Catarina, including the primary and secondary sectors in the production chain through production and commercialization of in natura grapes and the processing of raw material for the preparation of juice, wine and jams. The scenery in Santa Catarina has been changing in the last years, and traditional regions in grapes production have showed a decrease of the area under vines and a reduction in harvesting. Since the 1990s it is observed the decrease in vineyards productivity and the increase of plants mortality. Pathogenic fungi causing vascular wilt (Fusarium oxysporum f. sp. herbemontis) and root rot (Cylindrocarpon sp., Armillaria mellea, Rosellinia necatrix), ground-pearl (Eurhizococcus brasiliensis) and viruses are the main problems for the viticulture in the south of Brazil [1] [2] . Those factors together, known as grapevine decline, can lead vines to death due to the progressive weakening of the plants [2] .
Varietal resistance is one of the most indicated strategies to overcome those problems. Recently, Dalbó et al. [3] observed that IAC 571-6, IAC 572, IAC 313 and IAC 766 rootstocks from crossings with tropical species did not show plants declining or death, even where crops were produced in areas with plants death record and high level of ground-pearl infestation.
Other matters related to plant health in the South of Brazil are fungi diseases on shoots of the vine, especially downy mildew (Plasmopara vitícola) [4] . Heavy rainfall and high relative humidity throughout the year, mainly during growing and maturation stages can benefit the occurrence of that disease in Santa Catarina [5] . Under appropriate weather conditions for developing the pathogen, plant health treatments can represent 30% of vine production costs [6] . Thus, more consistent alternatives can be searched in order to enable vine cropping under the intrinsic weather conditions in southern Brazil.
The establishment of “Poloskei Muscotaly” cultivar aiming white wine production as well in natura fruit consumption has been advocating in Santa Catarina, due to its productive performance (above 20 t∙ha−1) and good tolerance to downy mildew and powdery mildew (Uncinula necator) [7] .
Phytosanitary conditions of mother plants, shoots and/or rootstock are directly linked to the capacity of the plant material in expressing all its genetic potential. The use of infected seedlings from other countries or other states in combination with the propagating method promotes the spread of diseases, mainly viruses [8] . In this sense, micropropagation techniques are important tools to obtain and maintain mother plants in high health quality. That process allows mass plant breeding and the formation of mother plants free from viruses, as well the material conservation in aseptic environment [9] -[11] .
This study had as objective to evaluate the establishment and in vitro multiplication of vine genotypes with potential in the south of Brazil, in different culture medium and ex vitro acclimatization.
2. Materials and Methods
Experiments were performed in Biotechnology Laboratory of EPAGRI-Empresa de Pesquisa Agropecuária e Extensão Rural de Santa Catarina (Company of Agriculture Research and Rural Extension of Santa Catarina), Experimental Station, in Lages/SC.
Mother plants from IAC 571-6 rootstock (Vitis caribaea × Pirovano 57) and from Poloskei Muscotaly canopy cultivar (Zalagyöngye × (Gloria Hungariae × Afuz ali)) were kept in greenhouse for providing explants.
Independent experiments were performed for each vine cultivar by a completely randomized design, with five treatments (saline formula) and thirty repetitions for each treatment in the evaluations during in vitro phase, and ten repetitions during acclimatization phase.
It was tested saline formulas by Galzy [12] , Roubelakis [13] , C2D [14] , DSD1 [15] and Zlenko [16] proposed for in vitro vine cultivation, supplemented with 20 g∙L−1 of sucrose and 7 g∙L−1 of agar, with no added growth regulators.
2-cm-nodal segments, with a single bud were used as explants source for in vitro cultivation. Under aseptic conditions, nodal segments were superficially sterilized, embedded in 70% alcohol (v/v) for 15 seconds and rinsed two times in sterile water; after, they were immersed in 80% sodium hypochlorite solution (v/v) and 0.1% Tween 20 (v/v) during 15 minutes and rinsed three times in sterile water. Explants were transferred into the test tubes (110 mm × 23 mm) with 10 mL of different culture media.
Cultures were maintained in the test room at 25˚C ± 2˚C during the fourth days in the dark, and after for a photoperiod of 16 hours of light per∙day?1 and luminous intensity of 50 μmol∙m?2∙s?1. Sub-crops were done each 45 days, segmenting sprouts in nodal segments of 1 cm length with a single bud. After the second sub-crop, in vitro cultures were maintained in growth for 60 days and were evaluated for number of leaves and roots, length of the bigger root and the aerial part, replication rate, chlorophyll index, dry matter from aerial parts and roots after drying in the oven at 60˚ for 48 hours, percentage of regenerated and rooted plants.
Replication rate was obtained by counting explants number which are originated when the material is replicated. For indirect measures of chlorophyll (chlorophyll index) in SPAD value, readings from adaxial face of the leaf located on the medium part of each bud, selecting completely expanded leaves through Handheld Chlorophyll Gauge SPAD-502 (Soil Plant Analysis Development, Konica Minolta®, Japan). Readings values performed with the gauge were calculated based on the quantity of transmitted light by the leaf on two wavelengths; the light that has passed through the leaf reaches a receptor which converts light on analog signals, and those ones on digital signals that are used to calculate SPAD values [17] .
Ex vitro acclimatization was performed with the third sub-cultures after 60 days in in vitro growing. Roots were washed in water and pruned (2 cm length); aerial part was maintained with 2 or 3 basal leaves. Buds were transferred to honey-combed trays with 72 cells (100 mL) containing sterilized substrate at 121˚C for 1 hour with Dystroferric Red Nitosol, sand and commercial substrate Tecnomax® (1:1:1, v/v/v). Trays were packed into plastic boxes, covered with glass and put in an acclimatization room during 60 days. Analyzed variables were the same as the experiments performed in vitro, exception to replication rate.
Statistical models were considered according to the nature of the variable response. For the variables Roots Number, Leaves Number and Replication Rate, it was used Poisson’s distribution. Model prepositions were verified using Kolmogorov-Smirnov’s Tests for normality of residuals, and Bartlett’s test for variance homogeneity.
In order to verify the model adjustment, it was used normal plot with simulated envelopes for deviance residual [18] . Data were submitted to variance analysis and, if significant, averages were compared by Tukey’s test at 5% significance level in R environment [19] .
3. Results and Discussion
In experiment 1, independent of the saline formula of each nutritive environment, in vitro cultures of Poloskei Mukotaly cultivar showed 100% of regeneration and rooting. Higher rooting rates were described by Biasi et al. [20] and Machado et al. [21] in in vitro micropropagation of Jales and VR 043-43 vine rootstocks, respectively, in formula without growth regulators.
Greater leaves quantity was found when Poloskei Muskotaly cultivar was cultivated in environments with Roubelakis’ and Galzi’s formula in relation to Zlenko’s one (Table 1). Values were superior to those showed by Biasi et al. [20] which displayed the influence of culture environments on leaves formation of in vitro vine cultures.
Roubelakis’ formulation promoted greater number of roots than C2D and DSD1 ones which induced higher mass accumulation of dry material; however, the culture environment did not affect the length of the longest root; the values were from 8.5 cm to 10.4 cm. When comparing MS [22] and Roubelakis’ formulations in micropropagation of 15 vine cultivars, Roubelakis-Angelakis and Zivanovitc [13] noted better development and greater formation of primary roots per culture with materials cultivated in Roubelakis’ environment. Results were similar to those found by Borghezan et al. [23] , who obtained, after 60 days of in vitro vine rootstocks cultivation, the greatest value of root length from 9.0 cm to 10.5 cm.
The longest aerial parts were found when Poloskei Muskotaly was cultivated in the middle of Roubelakis, with aerial part formation of 6.4 cm, not significantly differing from Galzy and DSD1 formulations with 5.5 cm and 5 cm, respectively (Table 1). These values are superior to the ones described by Villa et al. [24] and Barreto et al. [25] and inferior to those values found by Machado et al. [21] with vine rootstock multiplication in different culture environments without growth regulators.
Replication rate reflects the quantity of seedlings from a single explant. As a reflection of “number of leaves” and “stem length” variables, the higher replication rate of Poloskei Muskolaly cultures was in Roubelakis’ formulation, generating around 6.2 new plants, that do not differ significantly from cultures with Galzy, C2D and DSD1 formulations which, when replied, generated 5.6, 5.1 and 5.6 new plants, respectively (Table 1). Mukherjee et al. [26] determined influence of culture medium composition on in vitro development of deGrasset rootstock (Vitis champinii Planch.), and related formulas express effects on aerial growing rates which in turn are linked to the replication capacity of the inoculated explant.
In relation to chlorophyll index, values of SPAD-502 reading showed culture medium formulas influenced chlorophyll rate (Table 1). The highest chlorophyll indexes (IRC) were found when Poloskei Muskotaly cv. was cultivated on C2D and Roubelakis formulas (IRC around 27), differing significantly from de other culture medium; ZL was the same as Galzy’s and DSD1 did not differ from Galzy’s. Likewise, Guiñazú et al. [27] related the influence of culture medium on Criolla Grande and Pedro Giménez vine cultivars and the biggest values were found to Criolla Grande cultivated on  MS formula. According to the authors, IRC of Cereza, Criolla Chica and Torrontés Riojano cultivars in different culture medium have varied from 23.3 to 31.2, similar to those described for Poloskei Muskotaly in investigated formulas. Results in this study can be explained by the difference on mineral constitution of saline formulas; nitrogen and magnesium contents are found in the investigated formulations and those which show higher IRC have higher concentration of these nutrients in the formula. Nitrogen and magnesium are nutrients that have participation on chlorophyll molecule synthesis and structure, therefore when there is an increment of those nutrient sources, higher chlorophyll contents are observed [28] .
MS formula. According to the authors, IRC of Cereza, Criolla Chica and Torrontés Riojano cultivars in different culture medium have varied from 23.3 to 31.2, similar to those described for Poloskei Muskotaly in investigated formulas. Results in this study can be explained by the difference on mineral constitution of saline formulas; nitrogen and magnesium contents are found in the investigated formulations and those which show higher IRC have higher concentration of these nutrients in the formula. Nitrogen and magnesium are nutrients that have participation on chlorophyll molecule synthesis and structure, therefore when there is an increment of those nutrient sources, higher chlorophyll contents are observed [28] .
In vitro Poloskei Muskotaly cultures showed similar accumulation of dry mass among the treatments, with total biomass production in the interval from 39.92 mg to 50.82 mg, without statistical difference among culture medium formulations (Table 1). Values were higher than those related by Borghezan et al. [23] with SO4 and Paulsen 1103 vine rootstocks, which after 60 days of in vitro cultivation in DSD1 formula showed 34.8 mg and 35.6 mg dry mass accumulation, respectively.
In relation to the allocation of in vitro supplies, Poloskei Muskotaly showed around 80% of dry mass accumulation in the aerial part, without significant difference among the culture medium (Table 1). Results are in accordance with those by Ribeiro [29] , who observed higher accumulation of leaves and stem dry mass from Paulsen 1103 and VR 043-43 vine rootstocks and from Cabernet Sauvignon canopy in relation to roots dry mass. According to the author, higher accumulation of aerial dry mass was observed to Paulsen 1103 rootstock when cultivated in ME formula Torregrosa [30] with dry mass formation of 23.2 mg.
The largest root dry mass accumulation of Poloskei Muskotaly was obtained when cultivating in Roubelakis formulation, with 8.89 mg of dry roots, superior to DSD1 (6.17 mg) and Galzy (5.61 mg) formulas and without differing significantly from C2D and ZL with an accumulation around 8.00 mg of dry roots. Although not the best formula for root dry mass variable, Poloskei Muskotaly cultivated with DSD1 is between the limits of 2.7 mg and 7.6 mg of dry root as defined by Borghezan et al. [23] who evaluated six vine rootstocks in DSD1 formula.
For ex vitro acclimatization, Poloskei Muskotaly cultivar showed, after 60 days, 100% of survival independently of the culture medium used on in vitro propagation phase. In relation to the effects of acclimatization environment on survival rate of Jales’ vine rootstock, Biasi et al. [20] observed plant survival from 92.5% to 100% which were acclimatized in an environment under misting, in opened and closed containers, respectively. Moreover, Dzazio et al. [31] comparing different substracts for “420-vine” rootstock acclimatization, observed high survival rates regardless of the type of particulated substract. These researches and others relating high survival on vine acclimatization as Blazina et al. [32] and Lewandowski et al. [33] have showed ease vine adaptability on transferring in vitro to ex vitro medium.
After acclimatization, it was possible to detect effects of culture medium formulas on Poloskei Muskotaly cultivar plants (Table 2). Greater quantities of leaves are found on plants which were cultivated previously with Roubelakis, ZL, C2D and DSD1, and that last formula does not differ from Galzy. For “roots number” variable it was not observed statistical difference among formulas; Poloskei Muskolaly acclimatized plants form in average 2.8 to 4.4 roots per plant. In contrast, the longest roots were found when Poloskei Muskolaly cultivar was submitted to in vitro phase in ZL formula, with average length of 29.3 cm, superior to Galzy formula and not differing significantly from Roubelakis, C2D and DSD1 culture medium (Table 2). These results are superior to those found by Schuck et al. [34] evaluating different substracts for vine Bordô cultivar acclimatization, who obtained the best average roots lengths around 14.9 cm in Plantmax® substract; however, the evaluation was performed at 36 days of ex vitro cultivation.
Longer aerial parts of acclimatized Poloskei Muskolaly cultivar are found when being previously cultivated in Roubelakis, ZL, C2D and DSD1formulas, in an interval of 8.20 cm to 10.60 cm; however, Galzy substract was significantly inferior to Roubelakis and ZL formulas (Table 2).
IRC of acclimatized plants was lower than in vitro readings and it was not determined effects of culture medium in this phase, possibly because plants were submitted to the same nutritional conditions unlike in vitro phase (Table 2). Similarly, Borghezan et al. [23] observed that acclimatized plants from VR043-43, VR039-16 and Paulsen 1103 vine rootstocks showed lower chlorophyll content in relation to in vitro plants; authors have highlighted those plants are in an adjustment process to new environment conditions.
After 60 days of acclimatization, the accumulation of dry biomass was bigger for Poloskei Muskotaly plants
![]()
Table 1. Number of leaves and roots, length (cm) of the longest root and aerial part and replication rate (TR), relativachlorophyll index (IRC), dry mass (mg) of roots and aerial part, total biomass (mg), regeneration (R) and rooting (E) of Poloskei Muscotaly vine nodal segments cultivated in different culture medium.
Averages followed by the same letter in the column do not differ among them by Tukey test (p < 0.005).
![]()
Table 2. Number of leaves and roots, length (cm) of the longest root and aerial part, relativachlorophyll index (IRC), dry mass (mg) of roots and aerial part, total biomass (mg) and survival index (TS) of acclimatized Poloskei Muscotaly vine seedlings cultivated in different culture medium.
Averages followed by the same letter in the column do not differ among them by Tukey test (p < 0.005).
cultivated in vitro in Roubelakis, ZL and C2D formulas in relation to DSD1 and Galzy. This result shows that conditions of nutrients availability from culture medium in in vitro micropropagation phase have influence on plants growing in the posterior phase of ex vitro acclimatization, during heterotrophic and autotrophic conditions.
In respect of biomass allocation in different acclimatized plant organs, the greatest accumulation occurred on the aerial part and the best results for dry material from “aerial part” and “roots” variables followed the same order of total biomass (Table 2).
In experiment 2, in vitro cultures from IAC 571-6 rootstock showed greater growth of aerial parts and roots in Roubelakis and ZL culture medium. This is reflected on dry biomass accumulation; likewise, replication rate and leaves and roots number was superior in Roubelakis medium, followed by ZL (Table 3).
For “leaves number” variable, the greatest leaves quantity in IAC 571-6rootstock is found when it is cultivated in Roubelakis formula with a formation of 10.6 leaves per explant (Table 3). Formula effect on leaves number produced for in vitro vine explants were related by Villa et al. [24] , Dzazio et al. [31] and Biasi et al. [20] , and the results found with Roubelakis medium for “leaves number” variable are superior to those determined by the authors mentioned above.
Concerning the number of roots for IAC 571-6 rootstock, Roubelakis showed greater average with formation of 2.05 principal roots per plant, which did not differ from ZL formula (Table 4). Variations of genotype responses for different culture medium are related to the composition of nutrients formulas. Similarly, it was observed by Barreto et al. [25] , in a research of eight culture medium in in vitro Red Globe cultivar propagation, that distinct sources of nutrients have compounded formulas and have influenced on cultivar development.
Longer length of roots from IAC 571-6 rootstock was observed when cultivating in Roubelakis and ZL mediums, differing significantly from the others (Table 3). Results were superior to those found by Borghezan et al. [23] , who cultivated six vine rootstocks in DSD1culture medium, and inferior to those related by Silva et al. [35] after 60 days of Grav, Fercal, SO4 and Riparia rootstocks cultivation in DSD1 formula.
The best development of vine aerial part cultivars in Roubelakis formula was observed by Roubelakis-Ange- lakis and Zivanovitc [13] when comparing MS and Roubelakis formulas. Likewise, Zlenko et al. [16] obtained better development of Padorok Magaracha e Zhembhug Magaracha in vitro aerial parts cultivated in proper formula when comparing to 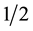 MS. Results from aerial part length of IAC 571-6 rootstock in Roubelakis and ZL formulas are similar to those related by the authors above. As a consequence, greater replication rates are found in Roubelakis culture medium generating 9.5 new plants, differing from the other formulas, followed by ZL generating 6.6 new plants, which differs from C2D, Galzy and DSD1, that in average formed 4.9 new plants at each bounce (Table 3). Formulations effect of culture medium on replication rate was highlighted by Krizan et al. [36] when propagating Kober 5BB, Kober 125AA e Teleki 5C vine rootstocks. It was verified all replication rates found for IAC 571-6 rootstocks were superior to those related by the author mentioned above.
MS. Results from aerial part length of IAC 571-6 rootstock in Roubelakis and ZL formulas are similar to those related by the authors above. As a consequence, greater replication rates are found in Roubelakis culture medium generating 9.5 new plants, differing from the other formulas, followed by ZL generating 6.6 new plants, which differs from C2D, Galzy and DSD1, that in average formed 4.9 new plants at each bounce (Table 3). Formulations effect of culture medium on replication rate was highlighted by Krizan et al. [36] when propagating Kober 5BB, Kober 125AA e Teleki 5C vine rootstocks. It was verified all replication rates found for IAC 571-6 rootstocks were superior to those related by the author mentioned above.
In relation to IRC, values for SPAD-502 readings revealed formulas from culture medium have influenced chlorophyll index (Table 3). Greater IRCs were found when IAC 571-6 rootstock was cultivated in C2D, DSD1 and Roubelakis formulas, with IRC from 22.6 to 25.7; Galzy formula did not differ from Roubelakis and ZL ones. These results are justified by the difference in mineral constitution of saline formulas; higher nitrogen and magnesium contents are found in formulas with greater IRCs. Similarly, as observed for Poloskei Muskotaly cultivar, IRC reading values from in vitro IAC 571-6 rootstock plants were superior to acclimatized plants (Table 4); so, it is evident that, according to estimated chlorophyll values in in vitro plants, they were not limiting to photosynthetic functioning.
For total biomass production of IAC 571-6 rootstock, the greatest dry mass accumulation was reached when rootstock was cultivated in Roubelakis and ZL formulas forming 69.8 mg and 57.2 mg of dry mass (Table 3). Accumulated values of total dry mass for IAC 571-6 rootstock, when cultivating in Roubelakis and ZL, were superior to those related by Ribeiro [29] and Silva et al. [35] in rootstocks and vine crown cultivars. However, those last authors related superior values of total dry mass accumulation (70.3 mg) for Grav rootstock.
In relation to in vitro biomass allocation, similarly to Poloskei Muskotaly cultivar, IAC 571-6 rootstock showed higher dry mass accumulation from the aerial part, representing in average 76% of the total (Table 3). Similarly, Borghezan et al. [23] demonstrated the greatest dry mass accumulation from the aerial part of in vitro vine plants, so that biomass accumulation takes place in larger quantity in leaves, followed by stem, and in roots in lower quantity. More tissue formation in aerial part has occurred when rootstock is cultivated in Roubelakis and ZL formulas. Greater dry mass accumulation from IAC 571-6 rootstock roots was obtained in Roubelakis formula, with 18.35 mg of dry roots, statistically superior to the other treatments (Table 4). According to Silva et al. [35] , Silva et al. [37] and Silva and Doazan et al. [15] , dry biomass, stem length and leaf area are among the more reliable parameters to evaluate in vitro development and multiplication of vine genotypes.
In vitro cultures of IAC 571-6 rootstocks expressed high potential of regeneration and rooting, whatever the culture medium used (Table 3), so that almost all in vitro explants regenerated excepting in C2D formula which
![]()
Table 3. Number of leaves and roots, length (cm) of the longest root and aerial part and replication rate (TR), relativachlorophyll index (IRC), dry mass (mg) of roots and aerial part, total biomass (mg), regeneration (R) and rooting (E) of IAC 571-6 vine nodal segments cultivated in different culture medium.
Averages followed by the same letter in the column do not differ among them by Tukey test (p < 0.005).
![]()
Table 4. Number of leaves and roots, length (cm) of the longest root and aerial part, relativachlorophyll index (IRC), dry mass (mg) of roots and aerial part, total biomass (mg) and survival index (TS) of acclimatized IAC 571-6 vine seedlings cultivated in different culture medium.
Averages followed by the same letter in the column do not differ among.
occurred the regeneration of 85% of inoculated nodal segments. However, all growing explants formed roots, so suggesting there is no need of growth regulator application which promotes rooting or specific phase for rooting. Similar results were found by Dzazio et al. [31] through nodal segments of “420-A” vine rootstock, which obtained rooting index near to 100% in NN [38] , Lloyd and Mccown [39] and MS/2 formulas, without growth regulators.
At the end of acclimatization phase, IAC 571-6 vine rootstock showed high index of survival similar to that obtained in other researches [23] [34] . Loss was verified only for C2D formula, in which just one acclimatized plant did not survive (Table 4).
Effects that culture medium carried out on in vitro development were not too expressive and evident in acclimatized plants (Table 4). Exception to “dry mass of aerial part” and “total biomass” variables, in which the greatest accumulation was observed when rootstock was previously cultivated in Roubelakis, ZL, Galzy and C2D formulas, and the last two ones did not differ from DSD1 formula. The relation between “dry mass from aerial part” and “dry mass from acclimatized vine plants roots” was research subject to Schuck et al. [34] ; the best proportion results for Bordô cv. were between 1.9 and 2.3. In this study, it was verified that all formulas showed superior results and proportion between 2.7 and 3.1, indicating that IAC 571-6 rootstock invests more in aerial part accumulation than Bordô cultivar.
Formulas of culture medium showed effects on morphological and physiological parameters of in vitro propagation and acclimatized vine cultivars. Multiplication rates and variant growth values of the cultivars demonstrated the importance of culture medium selection without growth regulators, since the addition of those ones to nutritive medium could not be favorable due to the induction of undesirable somaclonal mutations. Results in this research are in agreement with those found by Roubelakis-Angelakis and Zivanovitc [13] who demonstrated the influence of culture medium composition on the development of in vitro vine cultivars. Protocols of in vitro introduction and multiplication and acclimatization were applied successfully and showed higher indexes of survival, regeneration and rooting.
4. Conclusions
In vitro IAC 571-6 rootstock and Poloskei Muskotaly cultivar propagation through nodal segments obtained from mother plants maintained in greenhouse has promoted high indexes of regeneration and rooting.
In vitro root formation of IAC 571-6 rootstock and Poloskei Muskotaly cultivar has occurred in culture medium without growth regulators, and it is not needed a specific phase for root formation.
Considering all the analyzed variables, saline formula Roubelakis has promoted better growing and development of aerial parts and roots as well the best in vitro multiplication of IAC 571-6 and Poloskei Muskotaly vine cultivars.
Acknowledgements
Thanks to the Coordination of Improvement of Higher Education (CAPES) for the scholarship award to the first author at the Graduate School. We also thanks to the Company of Agriculture Research and Rural Extension of Santa Catarina for providing the structures for execution of the work.
NOTES
*Corresponding author.