Use of Ultrasound and Microwave Irradiation for Clean and Efficient Synthesis of 3,3’-(Arylmethylene)bis (2-hydroxynaphthalene-1,4-dione) Derivatives ()
1. Introduction
In reactions, quinones can act as nucleophiles or electrophiles depending on the electronic effects. Nucleophilic attacks occur at the carbonyl group or at the β-position through a Michael-like addition reaction. These reactions are favored by the electron-withdrawing groups in the quinone groups [1] [2] . Quinones acting as nucleophiles are less common and are favored by electron donor groups, such as hydroxy, methoxy and amino groups [3] .
Quinones are known to be electron transporters (e.g. ubiquinone, vitamin K) and are essential for many enzymatic processes [4] . They can act as anti- or pro-oxidants depending on the conditions of the media. Due to this chemical versatility they play important roles in different biochemical processes which are essential to living organisms [5] . Compounds with the quinone structure are of great importance due to their biological properties, their industrial applications and their potential as intermediates in the synthesis of heterocycles [6] . Quinone analogues of the coenzyme Q can selectively inhibit Plasmodium falciparum by adversely affecting the mitochondrial electron transport [7] . Studies have verified that the toxicity of naphthoquinones to Plasmodium sp. is due to interaction with some of the mitochondrial components of the respiratory chain [8] .
In this context, synthetic methods for the efficient preparation of new quinone derivatives present an attractive and interesting challenge.
In this paper, we report the use of a naphthoquinone, the 2-hydroxy-1,4-naphthoquinone (lawsone), acting as a nucleophile. Lawsone, also known as hennotannic acid, is the principal natural dye (1.0% - 1.4%) in the leaves of Lawsonia inermis (Lythraceae), commonly known as “henna” [9] . Multicomponent reactions using lawsone, aromatic aldehydes and different amines can be applied as a strategy for obtaining dyes and fluorescent compounds [10] .
To obtain synthetic derivatives of quinones it is important to use a sustainable synthetic methodology. For economic and environmental reasons, chemists constantly need to optimize their methods of synthesis. Currently, synthetic chemists are seeking alternatives that lead to the development of strategies to maximize the atom economy and minimize the costs and waste generation [11] . Solvent-free organic reactions have drawn considerable attention due to their environmentally acceptable protocols, short reaction times, occasionally enhanced selectivity and convenient means of product purification [12] [13] .
In this regard, sonochemistry has been rapidly developed in recent years. Its potential in environmental applications is drawing increasingly more attention [14] . Moreover, other physical impacts like heating and economic factors should also be considered, especially in practical scale-up systems. In general, as a part of a young and interesting scientific area, the application of ultrasound in environmental applications and green technology has a promising future. In particular, the beneficial effects of ultrasonic irradiation play an increasing role in chemical processes, especially in cases where classical methods require harsh conditions or prolonged reaction times, and ultrasound is considered to be an important tool for green chemistry in terms of waste minimization and energy conservation [15] .
An ideal synthesis method should, in principle, generate the desired product with 100% yield through of a reaction involving selectivity and a safe process. Herein, we report a rapid and facile procedure for the synthesis of 3,3’-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione) derivatives from lawsone and several aromatic aldehydes using ultrasonic and microwave irradiation, showing the importance of this reaction in terms of the concepts of atom economy while addressing environmental concerns and highlighting green chemistry.
2. Results and Discussion
The reaction between 2-hydroxy-1,4-naphthoquinone 1, acting as nucleophile, and the aldehydes 4-methox- ybenzaldehyde 2a, 3,4,5-trimethoxybenzaldehyde 2b, 4-bromobenzaldehyde 2c, benzaldehyde 2d, 3-bromo-2- hydroxybenzaldehyde 2e, 4-nitrobenzaldehyde 2f, 4-methylbenzaldehyde 2g, 4-fluorobenzaldehyde benzaldehyde 2h and 5-methylfuran-2-carbaldehyde 2i, first under ultrasonic irradiation and then applying microwaves in aqueous solution yielded 3,3’-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione) derivatives, 3a-3i. The synthesis of compounds 3-a to 3-i is shown in Scheme 1. Compounds 3-e and 3-i are previously unpublished. All synthesized compounds were obtained in good yields as shown in Table 1. The structures of all synthesized compounds were confirmed by IR, 1H NMR, 13C NMR and mass spectra data.
The characterization of these compounds is in agreement with data published in the literature [16] except for 3-e and 3-i. On analyzing the 1H NMR spectra, in general, in the region of higher chemical shift two sets of signals can be observed with four hydrogens for each integration. These signals refer to the hydrogens bonded di- rectly to the naphthoquinone cycle unit, which has two highly electrophilic centers (carbonyl group), contribut-
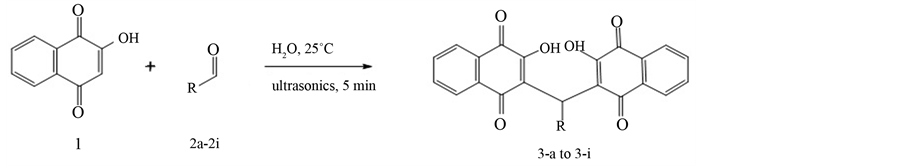
Scheme 1. Synthesis of compounds 3-a to 3-i.
![]()
Table 1. Physico-chemical data for 3,3’-(arylmethylene)bis(2-hydroxynaphthalene-1,4-dione) derivatives (3a-3i) in synthesis using ultrasound.
ing to the magnetic deshielded shift and higher frequencies. With regard to the other signals, in the region of lower chemical shift the presence of a singlet near 6.2 ppm integrated into a hydrogen for the aliphatic hydrogen can be observed, confirming the synthesis of the compounds. In the 13C NMR analysis, due to the symmetry of the compound, it was observed that some carbons exhibited the same chemical shift. Thus, the number of signals does not correspond directly to the number of carbon atoms present in the structure of each of the compounds. It is worth noting the presence of naphthoquinones carbonyls in regions of higher chemical shift (near 180 ppm), with enolic carbon near 155.0 and sp3 carbon near 37.1 ppm.
We performed the optimization of the reaction conditions for the synthesis of 3-a, 3,3’-((4-methoxy-phenyl) methylene)bis(2-hydroxynaphthalene-1,4-dione). Initially, microwave irradiation was applied under different conditions, that is, in aqueous and ethanolic medium, at temperatures of 55˚C, 70˚C and 85˚C and with different catalysts (aluminum chloride, indium (III) chloride, lithium chloride, sodium chloride, hydrochloric acid, ferric chloride, p-toluenesulfonic acid and potassium chloride) for 15 min (Table 2). The best results in terms of yield were obtained for the reactions performed using lithium chloride (78%) and indium chloride (76%) as catalysts in aqueous medium at 70˚C and 85˚C respectively. These conditions were therefore chosen to carry out the experiments with ultrasound and since the reaction yield using the two catalysts was very similar, lithium chloride was selected because it is cheaper. It should be noted that the aqueous medium used in these experiments is an environmentally innocuous solvent.
The reactions performed with ultrasound were carried out in an aqueous medium at room temperature. The reaction time was standardized at 5 min because this was the minimum interval for which we observed the formation of the products according to the thin layer chromatography analysis.
For comparison purposes, the reaction between lawsone and different aromatic aldehydes was carried out in two ways, one of them it was used a reflux system in water in the presence of lithium chloride for 12 h and the other it was applied microwave irradiation at 70˚C for 15 min.
The yields obtained for compounds 3a-3i in the synthesis using ultrasonic irradiation, microwave experiments and reflux, along with the percentage of atom economy, are shown in Table 3. The reaction using ultrasonic irradiation not only provided the best yields (Table 3), but was also successfully performed within a short reac-
![]()
Table 2. Reaction conditions for the synthesis of 3,3’-((4-methoxyphenyl)methylene)bis(2-hydroxynaphthalene-1,4-dione) (3a) using microwave experiments for 15 min.
![]()
Table 3. Comparison between the yields obtained in the synthesis of compounds 3-a to 3-i using different reaction methodologies.
aH2O, LiCl (catalyst), 12 h; bH2O, 70˚C, LiCl (catalyst), 15 min; cH2O, 25˚C, LiCl (catalyst), 5 min.
tion time (5 min) and at the lowest temperature. This contributes significantly to reducing the energy consumption making this methodology the most attractive of the three investigated.
The reaction between lawsone and the compounds 4-methoxybenzaldehyde (2a), 4-bromobenzaldehyde (2c), benzaldehyde (2d), 4-methylbenzaldehyde (2g) and 4-nitrobenzaldehyde (2f) was first studied by Tisseh and Bazgir [16] using reflux in aqueous media, in the presence of a catalytic amount of LiCl with reaction times of 12 - 24 h. These authors proposed a mechanism for this reaction in which the metal element (lithium) binds to the oxygen atom present in the aldehyde carbonyl leaving the more electrophilic carbon, which provides better conditions for the nucleophilic attack of lawsonate.
The conditions under which the ultrasonic irradiation reaction is performed are very important from the environmental point of view, since waste products are not generated, the only by-product of the reaction being water. One of the key principles of green chemistry is that processes should be designed so that the maximum amount of all the raw materials ends up in the product and a minimum amount of waste is produced. A reaction can have a high percentage yield while at the same time producing large amounts of waste products. The reactions observed in this study had a high atom economy. For compounds 3-a to 3-i the atom economy values obtained were very high (>96% for all compounds), which demonstrates the efficiency of this reaction and its environmental relevance (Table 3). Both the yield and the atom economy should be taken into account when designing a green chemistry process.
Moreover, the reaction using ultrasonic irradiation does not require the use of toxic solvents, the reaction time is short (decreasing the energy consumption), purification is easily performed and the procedure can be reproduced on a larger scale without loss of efficiency. It is noteworthy that the synthesis was performed using a natural compound, lawsone, which constitutes a renewable raw material. This reaction does not lead to the formation of undesirable by-products and a selective catalyst of low toxicity is used. The fact that toxic solvents and high temperatures are not required minimizes the risk of accidents, such as spills, fires and explosions.
Therefore, the use of ultrasound irradiation to synthesize the compounds studied herein is consistent with the principles of green chemistry (prevention, atom economy, less hazardous chemical syntheses, designing safer chemicals, safer solvents and auxiliaries, design for energy efficiency, use of renewable feed stocks, reduce derivatives, catalysis, real-time analysis for pollution prevention, inherently safer chemistry for accident prevention).
3. Experimental
3.1. Materials
The solvents used were all of analytical grade. The reagents 2-hydroxy-1,4-naphthoquinone, 4-methoxybenzal- dehyde, 3,4,5-trimethoxybenzaldehyde, 4-bromobenzaldehyde, 3-bromo-2-hydroxybenzaldehyde, benzaldehyde, 4-nitrobenzaldehyde, 4-methylbenzaldehyde, 4-fluorobenzaldehyde, 5-methylfuran-2-carbaldehyde, aluminum chloride, ferric chloride, lithium chloride, sodium chloride, hydrochloric acid, p-toluenesulfonic acid, potassium chloride and indium (III) chloride were obtained from Sigma Aldrich.
The compounds obtained were analyzed by thin layer chromatography (TLC) using aluminum plates coated with silica gel 60 GF254 (Merck). Infrared spectra (IR) were recorded using KBr on a Perkin Elmer 16 PC FTIR spectrometer. Melting points were recorded on the apparatus PF Chemistry MQA. 1H and 13C NMR spectra were obtained using a Varian AS-400 spectrometer operating at 400 MHz for 1H and 100 MHz for 13C, using acetone-d6 as the solvent (chemical shifts in ppm). The mass analysis was performed on an amaZon speed ETD Trap Mass spectrometer. A Milestone microwave (model Star Synth) was used, employing a power of 1200 Watts. The reactions were carried out with a microtip probe connected to a 500 W Sonics Vibracell ultrasonic processor operating at 20 kHz at 25% of the maximum power output.
3.2. Procedure for the Preparation of 3,3’-(Arylmethylene)bis(2-hydroxynaphthalene- 1,4-dione) Derivatives (3-a to 3-i) under Ultrasonic Irradiation
In a 25 mL beaker, 2-hydroxynaphthalene-1,4-dione (2 mmol), aldehyde (1 mmol) and LiCl (1 mmol) were mixed with water (5 mL) and sonicated for 5 min at room temperature (25˚C). The crude products were allowed to cool in a refrigerator. The precipitates obtained were filtered and washed with water and then with EtOH to afford the pure product (3-a to 3-i). The reaction progress was monitored by TLC.
3.3. Procedure for the Preparation of 3,3’-(Arylmethylene)bis(2-hydroxynaphthalene- 1,4-dione) Derivatives (3-a to 3-i) under Microwave Irradiation
In a flask, 2-hydroxynaphthalene-1,4-dione (2 mmol), aldehyde (1 mmol) and LiCl (1 mmol) were mixed with water (5 mL) under microwave irradiation at 70˚C for 15 min. The reaction progress was monitored by TLC. The precipitates obtained were filtered and washed with water and then with EtOH to afford the pure product (3-a to 3-i).
3.4. Procedure for the Preparation of 3,3’-(Arylmethylene)bis(2-hydroxynaphthalene- 1,4-dione) Derivatives (3-a to 3-i) under Reflux
A mixture of 2-hydroxynaphthalene-1,4-dione (2 mmol), aldehyde (1 mmol) and LiCl (1 mmol) in refluxing water (5 mL) was stirred for 12 h. The reaction progress was monitored by TLC. After completion of the reaction, the reaction mixture was filtered and the precipitate washed with water and then with EtOH to afford the pure product 3a-3i.
3.5. Spectral Characterization and Mass Analysis of Synthesized Compounds (3-a to 3-i)
3,3’-((4-methoxyphenyl)methylene)bis(2-hydroxynaphthalene-1,4-dione) (3-a):
m.p. 220˚C - 222˚C (same as reference [16] ); IR (KBr, cm−1): 3395 (O-H), 3249 (C-H Ar), 1665 (C=O), 1637 (C=O). 1H NMR (400 MHz, C3D6O): H 3.77 (3H, s, OCH3), 6.17 (1H, s, CH), 6.80 (2H, d, J = 8.8 Hz, H-Arom.), 7.72 (2H, d, J = 8.9 Hz, H-Arom.), 7.67 - 8.11 (8H, m, H-Arom.) ppm; 13C NMR (100 MHz, C3D6O): 37.1; 55.2; 113.76; 122.9; 126.3; 127.2; 129.6; 132.2; 135.0; 154.6; 158.4; 181.3; 184.7 ppm; MS (m/z, M-H, %): 465.21 (100).
3,3’-((3,4,5-trimethoxyphenyl)methylene)bis(2-hydroxynaphthalene-1,4-dione) (3-b):
m.p. 226˚C - 227˚C (reference [17] : m.p. not determined); IR (KBr, cm−1): 3400 (O-H), 3247 (C-H Ar), 1665 (C=O), 1637 (C=O). 1H NMR (400 MHz, C3D6O): 3.92 (3H, s, OCH3), 3.82 (3H, s, OCH3), 3.68 (3H, s, OCH3), 6.24 (1H, s, CH); 7.25 (2H, s, H-Arom.), 7.78 - 8.09 (8H, m, H-Arom.). 13C NMR (100 MHz, C3D6O): 37.4; 55.5; 114.1; 123.2; 126.6; 127.5; 129.9; 133.1; 133.5; 135.3; 155.0; 158.7; 181.6; 185.6; 185.0; MS (m/z, M-H, %): 525.20 (100).
3,3’-((4-bromophenyl)methylene)bis(2-hydroxynaphthalene-1,4-dione) (3-c):
m.p. 216˚C - 217˚C (216˚C - 218˚C in reference [16] ); IR (KBr, cm−1): 3396 (O-H), 3248 (C-H Ar), 1666 (C=O), 1636 (C=O). 1H NMR (400 MHz, C3D6O): 6.17 (1H, s, CH); 6.83 (2H, d, J = 9.03 Hz, H-Arom.), 7.21 (2H, dd, J = 1.0 and J = 8.03 Hz, H-Arom.), 7.68 - 8.12 (8H, m, H-Arom.). 13C NMR (100 MHz, C3D6O): 36.7; 113.5; 122.6; 126.0; 128.9; 129.3; 132.4; 132.9; 134.7; 154.3; 158.1; 181.0; 184.4; MS (m/z, M-H, %): 515.18 (100).
3,3’-(phenylmethylene)bis(2-hydroxynaphthalene-1,4-dione) (3-d):
m.p. 202˚C - 204˚C (same as reference [16] ); IR (KBr, cm−1): 3360 (O-H), 1672 (C=O), 1644 (C=O). 1H NMR (400 MHz, C3D6O): 6.11 (1H, s, CH); 6.77 (2H, d, j = 9.03 Hz, H-Arom.), 7.15 (2H, dd, J = 1.0 and J = 9.03 Hz, H-Arom.), 7.61 - 8.06 (8H, m, H-Arom.). 13C NMR (100 MHz, C3D6O): 37.1; 1138; 122.9; 127.2; 129.3; 129.6; 132.7; 135.0; 154.6; 158.4; 181.3; 184.7; MS (m/z, M-H, %): 535.30 (100).
3,3’-((3-bromo-2-hydroxyphenyl)methylene)bis(2-hydroxynaphthalene-1,4-dione) (3-e):
m.p. 215˚C - 217˚C; IR (KBr, cm−1): 3396 (O-H), 3250 (C-H Ar), 1666(C=O), 1636 (C=O). 1H NMR (400 MHz, C3D6O): 6.11 (1H, s, CH); 6.77 (2H, d, J = 8.78 Hz, H-Arom.), 7.15 (2H, dd, J = 0.75 and J = 828 Hz, H-Arom.), 7.61 - 8.06 (8H, m, H-Arom.). 13C NMR (100 MHz, C3D6O): 37.1; 113.8; 122.9; 127.2; 129.3; 129.6; 132.7; 133.2; 135.0; 154.6; 158.4; 181.3; 184.7; MS (m/z, M + H, %): 531.13 (100).
3,3’-((4-nitrophenyl)methylene)bis(2-hydroxynaphthalene-1,4-dione) (3-f):
m.p. 177˚C - 178˚C (177˚C - 179˚C in reference [16] ); IR (KBr, cm−1): 3401 (O-H), 1666 (C=O), 1636 (C=O). 1H NMR (400 MHz, C3D6O): 6.11 (1H, s, CH); 6.77 (2H, d, J = 9.03 Hz, H-Arom.), 7.15 (2H, dd, J = 1.00 and J = 9.03 Hz, H-Arom.), 7.63 - 8.06 (8H, m, H-Arom.). 13C NMR (100 MHz, C3D6O): 36.7; 113.4; 126.0; 126.9; 128.9; 129.3; 132.4; 132.9; 134.7; 154.3; 158.1; 181.0; 184.4; MS (m/z, M-H, %): 480.12 (100).
3,3’-(p-tolylmethylene)bis(2-hydroxynaphthalene-1,4-dione) (3-g):
m.p. 170˚C - 172˚C (same as reference [16] ); IR (KBr) (νmax, cm−1): 3396 (O-H), 3249 (C-H Ar), 1666 (C=O), 1636 (C=O). 1H NMR (400 MHz, C3D6O): 6.59 (1H, s, CH); 6.60 (1H, s, CH); 7.16 - 7.19 (4H, m, H-Arom.); 7.51 - 8.15 (14 H, m, H-Arom.). 13C NMR (100 MHz, C3D6O): 19.9; 37.0; 115.6; 126.5; 127.1; 128.1; 128.8; 132.1; 133.5; 135.4; 138.6; 153.0; 158.8; 181.1; 184.3; MS (m/z, M-H, %): 449.34 (100).
3,3’-((4-fluorophenyl)methylene)bis(2-hydroxynaphthalene-1,4-dione) (3-h):
m.p. 194˚C - 195˚C (same as reference [18] ); IR (KBr) (νmax, cm−1): 3396 (O-H), 3248 (C-H Ar), 1667 (C=O), 1636 (C=O). 1H NMR (400 MHz, C3D6O): 6.18 (1H, s, CH); 6.83 (2H, d, J = 8.60 Hz, H-Arom.), 7.21 (2H, d, J = 8.60 Hz, H-Arom.), 7.68 - 8.12 (8H, m, H-Arom.). 13C NMR (100 MHz, C3D6O): 37.1; 113.8; 122.9; 127.2; 129.3; 129.6; 132.7; 135.0; 154.6; 158.4; 181.3; 184.7; MS (m/z, M-H, %): 453.11 (100).
3,3’-((5-methylfuran-2-yl)methylene)bis(2-hydroxynaphthalene-1,4-dione) (3-i):
m.p. 182˚C - 183˚C; IR (KBr) (νmax, cm−1): 3396 (O-H), 3249 (C-H Ar), 1666 (C=O), 1636 (C=O). 1H NMR (400 MHz, C3D6O): 2.53 (3H, s, CH3), 6.59 (1H, s, CH); 7.70 (2H, dd, J = 1.25 and J = 7.53 Hz, H-Arom.); 7.97 (3H, m, H-Arom.); 8.14 (4H, m, H-Arom.). 13C NMR (100 MHz, C3D6O): 17.9; 38.1; 111.0; 111.3; 117.9; 118.7; 123.7; 130.2; 130.7; 135.6; 139.4; 153.6; 156.7; 158.2; 181.5; MS (m/z, M-H, %): 439.13 (100).
4. Conclusion
In this study, we developed a mild, highly efficient and improved protocol for the preparation of 3,3’-(arylme- thylene)bis(2-hydroxynaphthalene-1,4-dione) derivatives under ultrasonic irradiation and in microwave experiments. Compounds 3-e and 3-i are previously unpublished. Our sonochemical method offers several advantages over existing methods, including improved yields, cleaner reactions, simple work-up and very short reaction times, making it a useful and environmentally attractive strategy for the synthesis of lawsone derivatives.
Acknowledgements
We would like to thank the Center of Analysis of the Department of Chemistry, Federal University of Santa Catarina, CEBIME and CAPES/CNPq for financial support.
NOTES
*Corresponding author.