Induction of Necrotizing Enterocolitis in Non-Premature Sprague-Dawley Rats and the Effect of Administering Breast Milk-Isolated Lactobacillus salivarius LPLM-O1 ()
1. Introduction
Necrotizing enterocolitis (NEC) is an extremely catastrophic and common gastrointestinal disease acquired by very low birth weight infants (VLBW, with birth weight being under 1.5 kg). The incidence of NEC varies from country to country, affecting from 2.6% to 28% of VLBW infants, and its associated mortality goes from 16% to 42% [1] . Since NEC usually goes from early signs of intestinal inflammation to severe necrosis in just hours, establishing prevention strategies is preferable to determining treatments for the pathology. A total of 18 clinical tests, 9 meta-analysis and systematic reviews have presented conclusive results on the effectiveness and security of oral probiotics in preventing NEC and reducing its mortality [2] [3] .
Developing reliable animal models that can be reproduced for studying NEC remains a key part of the attempts to determine its main causes. In this context, studies based on surgical procedures in infants with NEC have shown limited value, as the extirpated tissue samples usually show necrosis and non-specific inflammatory changes; thus, they are not useful for studying the early events leading to NEC. In vivo animal models would be the best option for studying this disease hence. Several animal models have been used in the study of NEC, such as acute intestinal injury models, where the injuries are induced by intravascular administration of platelet-activating factors (PAF), on young adult rats [4] and mice [5] . Recently, a neonatal model of NEC on caesarean-born, formula-fed mice that were daily exposed to stress by hypoxia has been developed [6] . In this model, mothers must be sacrificed during the birth, which means that the control pups used in biochemistry studies, which are breast-fed, are obtained from different litters, and that the mothers cannot be used more than once—a problem when working with the expensive genetically-manipulated mice. Besides, caesarean-section is linked to a high mortality in newborn mice that are not related to NEC.
To optimize the study models, Tian et al. (2010) proposed the use of vaginally born pups, giving them maternal care for 12 hours and including a quick inoculation of commensal bacteria after birth, and so increasing the incidence of NEC in C57BL/6 mice [7] . When inoculating the pups with 105 CFU of E. fecalis, then exposing them to stress by hypoxia and cold, and feeding them with formula, a severe incidence of NEC was seen (37%) when compared to the control group (6%). The advantages of the model lie on dispensing with caesarean-born animals and favoring vaginally-born pups for the induction of NEC.
This study suggests modifying the model for artificial induction of NEC in mice. The proposed model uses Sprague-Dawley rats, vaginally born and with 48 hours of maternal care, which are then fed with formula, to induce NEC by hypoxiaand temperature-caused stress on them. The effects of the oral administration of the probiotic strain Lactobacillus salivarius LPLM-O1 are also studied in this model.
2. Materials and Methods
2.1. Microorganisms
The probiotic strain Lactobacillus salivarius LPLM-O1 was isolated from breast milk and identified through ribotyping, using primers 16SLacA (GAGAGTTTGATCCTGGCTCAG) and 16SLacB (CTACGGCTACCTTGTTACGA). Then, powdered microorganism biomass was treated to obtain the final solution, oily drops whose probiotic concentration (active substance) was 109 CFU/g.
2.2. Animal Model
Sprague-Dawley rats were bred to obtain pups, which were used as study subjects in the NEC model. Pregnancy was checked by an echographic analysis. 26 vaginally-born pups (after 21 days of pregnancy) were collected and kept in maternal care for 48 hours; then, they were put in incubators with controlled temperature and humidity, and fed with milk formula Similac NeosureTM, Abbott (daily dose of 200 kcal/kg). Chosen feeding method was oral feeding, with a silicone catheter and a syringe. The amount of milk given to each pup was 0.1 ml every 3 hours, during 4 days (96 hours). Animals were then randomized to form the experimental groups, 1) NEC group (n = 10), given a placebo (milk) and then NEC was induced; 2) probiotic group (n = 10), treated with L. salivarius LPLM-O1 (109 CFU/day) every 3 hours during the whole experiment and then induced to NEC; and 3) control group (n = 6), only given the placebo. NEC was induced with the method described by Caplan et al. in which the pups are fed with formula and then stressed through hypoxia (exposition to 100% gaseous nitrogen for 50 seconds) and cold (4˚C for 10 minutes) twice per day. The animals that suffered cyanosis, abdominal distension, breathing distress or lethargy were sacrificed using CO2 anaesthesia and decapitation. Animals alive at 96 hours were also sacrificed; their intestines were extracted for evaluating NEC and microbiological counting. The protocol was approved by the Ethics Committee of the University of Concepción.
2.3. NEC Evaluation
After the sacrifice, the rats’ intestines were macroscopically evaluated to check the presence of necrosis and were carefully removed and fixed in 10% neutral buffered formalin for 48 hours, embedded in paraffin, and sectioned at a thickness of 7 µm. Sections were stained with hematoxylin and eosin for the subsequent histological analysis. The histological findings were evaluated according to standard punctuation, where 0 = no necrosis, 1 = mild with epithelial sloughing, 2 = moderate with midvillous necrosis, 3 = severe with complete villous necrosis, and 4 = transmural necrosis [8] .
2.4. Microbiological Analysis
To do the microbiological analyses, a portion of each pup’s small intestine was collected in microcentrifuge tubes and mechanically homogenizated in saline solution. The samples were then serially diluted in saline solution and sown in MRS agar plates, which were incubated in microaerophilic conditions at 37˚C for 24 - 48 hours. The results were expressed as CFU/g tissue.
2.5. Statistical Analysis
The Kaplan-Meier method was applied to estimate survival probability and log-rank tests to compare the survival curves among groups. The Mann-Whitney U test was used to compare body weight among the study and control groups. All analyses were performed using Prisma 5 statistics software (GraphPad Software, Inc., California, USA).
3. Results
At macroscopic level, the tissues of the NEC-induced animals looked friable, oedematous and with gas inside them, which reflects the pups’ abdominal distension. At histological level, the NEC-induced animals showed damage mainly in the ileum, but not in the duodenum; all animals presented epithelial damage, and in most cases, the villi was completely damaged (Figure 1(c), black arrows), with presence of red blood cells among the rests of lamina propria tissue (Figure 1(c), black arrowhead). In the severe damage of the intestinal mucous, were observed clusters of erythrocytes in the intestinal lumen. Those animals treated with the probiotic strain and subjected to induction of NEC displayed moderate histologic changes in the epithelium, which included presence of micro vacuoles in the epithelial enterocytes. Those damages are not enough to alter cell shape, but they could alter its function (Figure 1(b), red arrows).
The pups of the control group did not show changes in the intestine, which presented normal characteristics (Figure 1(a)).
The survival rates recorded throughout the assay were 46% for the NEC-induced pups, 83% for the probiotic group, and 100% for the control group (Figure 2), however, the difference was not statistically significant (p = 0.14).
Regarding the weight lose, all groups presented an important weight loss; however, the probiotic group lost the less weight, with a 24.9% loss in the 96 hours of the assay (Table 1). However, weight variation is not
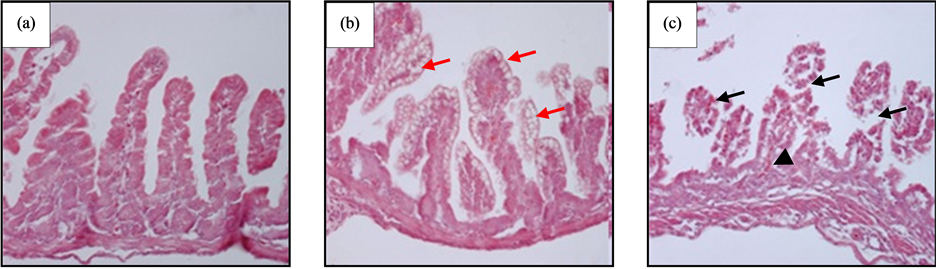
Figure 1. Histological analysis of ileum, stained with hematoxylin-eosin. (a) Negative control, no evident histological alterations; (b) Probiotic group, showing epithelial cells with cytoplasmic microvacuoles or possible increase of goblet cells; (c) NEC group, showing damage in all mucosas, no visible villus, only rests of base connective tissue of the lamina propria. Red arrows: epithelial microvacuoles; black arrows: damage in intestinal villous; black arrowhead: red blood cells in lamina propria. Original magnification ×100.
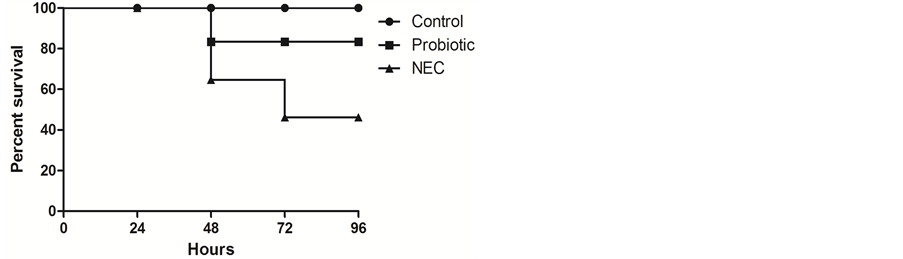
Figure 2. Kaplan-Meier survival curve of NEC-induced pups during the 96 hours of the assay.

Table 1. Body weight variation of the pups.
Animals of the NEC group (n = 10), given a placebo (milk) and then NEC was induced; (2) probiotic group (n = 10), treated with L. salivarius LPLM-O1 (109 CFU/day) every 3 hours during the whole experiment and then induced to NEC, and (3) control group (n = 6), only given the placebo, were weighed at the beginning and end of the experiment and the weight loss calculated.
significant among the three groups.
Regarding the weight lose, all groups presented an important weight loss; however, the probiotic group lost the less weight, with a 24.9% loss in the 96 hours of the assay (Table 1). However, weight variation is not significant among the three groups (p > 0.05).
In the macerated intestine sowings done in MRS medium, there was a concentration of 6.1 × 107 CFU of lactic acid bacteria per intestinal tissue gram in the NEC group, and a concentration of 8.4 × 108 CFU/intestinal tissue gram in the probiotic group.
4. Discussion
Until recently, there were no suitable neonatal study models for NEC and the researchers were limited to use murine models of acute intestinal damage in young animals, whether induced by ischemia-reperfusion injury [9] or by the incorporation of PAF [5] . However, these models did not consider the differential characteristics on the premature newborns with risk of NEC. Julling et al. (2006) developed a neonatal model of NEC in mice, in which newborn mice were obtained by Caesarea and exposed to formula feeding and stress by hypoxia-cold [6] . This model resulted in a variable incidence of NEC and a high rate of mortality, the latter associated to birth by Caesarea. Later, Tian et al., in a neonatal mice model of NEC, demonstrated that vaginally-born mice, fed with formula and allowed maternal contact for 12 hours, which were artificially provided with commensal bacteria showed an increased NEC incidence, generating a more reproducible model [7] . The authors stated to not have found any protective effect that could be attributed to vaginal birth, in contrast with Caesarea, in the incidence or severity of NEC in the neonatal model. This is consistent with what is known of human NEC, where there are no studies showing a clear relationship of the pathology with the type of birth [10] . Besides, vaginal birth is preferred due to minimizing the potential adverse effects of chirurgical procedures, such as an excessive mortality by respiratory syndrome, apnea, and infection, all of them factors that can complicate data interpretation. The model covered also the artificial bacterial colonization by incorporating bacterial inoculates of adult commensal microbiota or the bacterium E. fecalis.
It has been proposed that indigenous intestinal microbiota has a key role in the pathogenesis of NEC [11] [12] , even if a higher incidence of NEC in microbiologically-induced neonatal rat models has been reported [6] . The artificial incorporation of microbiota could have some incidence on the model and affect its reproducibility, due to the change and composition of the microbiota.
In general, it has been described that to simulate the human condition it is necessary to choose an animal species whose pharmacokinetic and toxicokinetic mechanisms have been established and are similar to those of humans. The advantages of using rats in tests of chemical toxicity and drugs include a) metabolic pathways similar to the human ones; b) similar physiological and anatomical characteristics; c) a big data base, which is fundamental for comparison purposes; and d) easy breeding and maintenance of the animals at a relatively low cost. In this context, and as another way of optimizing the NEC model, Sprague-Dawley rats were used instead of C57BL/6 mice, the main reason being the considerably bigger size of the offspring at birth, a characteristic that highly facilitates the feeding and stimulation process, lessens the possibility of damage when manipulating them, and makes easier the dissection and extraction of tissues of better quality.
In this work, a study model was established, in which the pups are with their mothers for 48 hours, a time in which the mother’s microbiota could naturally reach its child, either by feeding or simply by body contact. All animals from the NEC group showed histological damage, mainly in the ileum, corresponding to a transmural necrosis (grade 4), according to Tian et al. [7] . The rats’ clinical status and their intestines’ macroscopic appearance were also considered for this model, as proposed by Zani et al. [13] . With this established and functional NEC model, the effect of the probiotic strain Lactobacillus salivarius LPLM-O1 was tested in NEC-induced Sprague-Dawley pups. First, the histological damage in the NEC-induced and probiotic-treated animals was determined to be lesser according the comparative scale, reaching a damage level of 2 - 3, which is less than that observed in the only NEC-induced individuals. The considerable histological damage in the latter group could have caused some alteration of the gastrointestinal tract, which might have triggered a higher weight loss in them. What is more, although the data was not statistically significant, the survival rate of the probiotic-fed pups was considerably higher. The efficacy of administering different probiotic strains to prevent NEC has been widely proved. [14] . Lactobacillus rhamnosus HN001 lowers the severity of the disease; activating Toll-like receptors (TLR9) is essential to obtain such an effect [15] . The administration of a probiotic mix containing Bifidobacterium bifidum and B. longum was effective in preventing death and NEC in a model with premature pups [2] .
In this context, the lactic acid bacteria counts done in the tract of NEC-induced pups on this model were 6.1 × 107 CFU/tissue g for the NEC group and 8.4 × 108 CFU/tissue g for the probiotic-fed group respectively, which proves that the not-artificially-inoculated pups show a barely lower bacterial colonization of the tract than those treated with probiotics, confirming the transfer of microbiota from the mother to the baby.
This model of induction mainly based in formula feeding and the application of hypoxia and temperature-induced stress in vaginally-born Sprague-Dawley pups that are fed by and in contact with their mothers for 48 hours, manages to reproduce the clinical signs associated to NEC at a macroscopic level, generating histological damage and mortality. Treating the pups with the probiotic strain Lactobacillus salivarius LPLM-O1 would reduce the epithelial damage and lower the mortality rate by NEC in what is an efficient and easily implemented study model; however, these findings must be corroborated.
NOTES
*Induction of necrotizing enterocolitis and effect of the probiotic strain LPLM-O1 in non premature rats.
#Corresponding author.