1. Introduction
The Mediterranean fruit fly (Medfly), Ceratitis capitata (Wiedemann), and the native South American fruit fly, Anastrepha fraterculus (Wiedemann) are among the major pests of fruit crops in Argentina. The occurrence of these two tephritid species have depicted a constraint for exporting fresh fruits to countries free of these pests or with active fruit fly control programs. Given this background, the National Agri-Food and Animal Health and Quality Service of Argentina has made the fight against these two pests a priority through implementing of the National Fruit Fly Control and Eradication Program (ProCEM) [1] .
The presence of tephritid fruit flies of economic importance in Argentina varies in different fruit-growing regions. For example, due to the climatic conditions in the temperate fruit-producing areas of the provinces of Mendoza and San Juan, located in the central-eastern region of Argentina known as Cuyo, the only fruit fly species that produce phytosanitary damage is C. capitata [1] . In San Juan, the strategies applied against Medfly through the ProCEM, jointly with the provincial government and producers, have been based on an integrated use of SIT (sterile insect technique), cultural and chemical controls [2] . However, in 2008 biological control was incorporated into activities of ProCEM-San Juan by establishing a laboratory colony of the exotic parasitoid Diachasmimorpha longicaudata (Ashmead) at BioPlanta San Juan mass-rearing facility [3] . This is the first facility in Argentina to produce D. longicaudata on a large scale. As a result of this production, currently 200,000 parasitoid adults are produced weekly in the BioPlanta San Juan facility using C. capitata larvae of a genetic sexing strain of a temperature-sensitive lethal (tsl) Vienna 8 strain [4] .
The Indo-Pacific species D. longicaudata is a larval-prepupal, koinobiont, solitary endoparasitoid of tephritid fruit fly species [5] . It was introduced throughout much of Latin American and in southern United States [5] . Currently, D. longicaudata is considered as one of the most important biological control agents for augmentative releases against pestiferous Anastrepha species and C. capitata in tropical and subtropical America [6] . This braconid parasitoid was selected for augmentative biological control programs in San Juan because it has revealed a high adaptability to the different environments into which it has been introduced [7] -[9] , good capacity for successful development on the larvae of C. capitata [10] , and by its host-searching ability at different hostdensities on a wide variety of fruit species at canopy and ground levels [11] -[13] . In addition, efficient techniques for mass rearing and augmentative releases of D. longicaudata have been developed in Hawaii [7] [9] and México [8] [14] [15] .
The objective of this study was to assess the effectiveness of laboratory-reared D. longicaudata females in order to find and successfully parasitize Medfly larvae in different host fruit species once released under semiarid conditions in ecologically isolated fruit-growing valleys of San Juan. In such semi-arid central-western areas of Argentina, the natural rates of parasitism on C. capitata are either extremely low or nil [4] . This research is part of a new activity of ProCEM-San Juan that includes the use of environment-friendly strategies to suppress or eradicate C. capitata in fruit-producing irrigated-valleys, such as releases of D. longicaudata in combination with sterile Medfly releases [3] .
2. Material and Methods
2.1. Importation of Parasitoid
The parasitoid D. longicaudata was obtained from a colony that had been maintained on larvae of Anastrepha ludens (Loew) at the Biological Control Laboratory of Mexico’s Moscamed-Moscafrut National Program and imported to Argentina within parasitized A. ludens pupae in 1999. All pupae were obtained from irradiated A. ludens larvae [16] . The shipment was immediately brought to the quarantine facility at the Center of for Research on Population Regulating Harmful Organisms in San Miguel de Tucumán, northwestern Argentina. Initially, D. longicaudata was maintained on larvae of a wild C. capitata strain under restricted conditions [10] . Several generations later, the parasitoid colony was transferred to the Biological Control Division of the Pilot Plant of Industrial Microbiological Processes and Biotechnology (PROIMI) in San Miguel de Tucumán. In the PROIMI, D. longicaudata was successfully reared on both late-third instars of C. capitata and A. fraterculus [17] . Then, shipments of D. longicaudata were sent to the “BioPlanta San Juan” facility between April and December 2008 for parasitoid mass rearing [3] . This factory belongs to the Directorate of Plant Health, Animal and Food of the government of San Juan province, located in San Juan city, central-western Argentina.
2.2. General Insect Rearing Conditions
Diachasmimorpha longicaudata was reared on third-instar larvae of VIENNA 8 TSL C. capitata strain in the Parasitoid Rearing Laboratory at “BioPlanta San Juan” facility. Adult parasitoids were kept in rectangular iron-framed, mesh-covered cages (0.5 × 0.5 × 0.6 m) holding 2000 pairs per cage at 24˚C ± 1˚C; 65% ± 5% RH and a photoperiod of 12:12 (L:D). Parasitoids were daily provided with honey, soaked in paper towels on the top of Petri dishes, and with small glasses that held wet cotton. Twice a day, each parasitoid cage was provided with eight oviposition units. Each oviposition unit was composed of an organdy screen-covered dish (10 cm diameter and 1 cm deep) and it contained about 1000 host larvae. These units were placed on the top of the cage. After exposure to the parasitoids, oviposition units were removed from the cages. Parasitized C. capitata larvae were then placed in emergence cups (70 cm diameter, 80 cm deep) with meshscreen covers and containing wheat bran as the pupation medium on the bottom. The cups were kept under the above mentioned laboratory conditions until the emergence of adult parasitoids. The larvae of TSL C. capitata strain were reared in the Medfly Rearing Laboratory at “BioPlanta San Juan” facility on wheat-based diet fortified with yeast, poplar shaving, sugar, hydrochloric acid, sodium benzoate, nipagin and water [3] .
2.3. Study Sites
The province of San Juan is characterized by fruit-producing areas focused on irrigated valleys, commonly named Oases. In these valleys climate is continental desert with important annual variation in temperature and atmospheric pressure. The annual temperature is 17.2˚C and annual rainfall is 165 mm. Precipitation is relatively moderate occurring mostly in summer (December-March). Six of these fruit-producing valleys, where there were host fruit plants highly infested by Medfly, were selected for parasitoid releases. Medfly host plants area scattered in backyards, small orchards, and also in extensive crops These Oases specialize in producing temperate fruits, mainly grape, quince, peach, plum, apricot, nectarine, olive, fig, as well as some citrus species are grown in small number. Further details on urban characteristics, cropped area, geographical coordinates, and altitude of each parasitoid release and collection site are provided in Table 1.
2.4. Environmental Conditions
Rainfall and maximum and minimum temperatures recorded in the study region were provided by the local weather station in the Pocito locality administered by EEA-INTA (Agricultural Experiment Station - National Institute of Agricultural Technology). Maximum temperature was monitored at 1500 hours and minimum temperature at 0600 hours.

Table 1. General description of parasitoid release and collection sites in San Juan, Argentina.
2.5. Parasitoid Releasing
Releases of D. longicaudata started in February 2009 and ended in June 2009. Between 2 and 4 parasitoid releases were performed at the study sites on different dates covering summer and autumn. Adult parasitoids were collected from colony 5 days after emergence, and transferred into paper bags with honey as food, at a capacity of 500 pairs per cage. The female:male sex ratio was 1:1. All release bags were placed into plastic containers, and these were transported to the release sites in air-conditioned vehicles. The bags were opened at each release place and a paper towel soaked in honey was put on a tree branch. Release densities fluctuated between 500 - 1000 adults per place. The releases were carried out at places with various Medfly host plants bearing fruits and in which no insecticides were regularly applied, preferentially in backyards, or in small orchards. Parasitoids were released using ground release-systems. The number of releases, release dates, and the number of parasitoid released for each study site are presented in Table 2. Figure 1 shows environmental conditions (mean daily rainfall and temperature) that encompassed this study during releasing months (February [middle summer], March [late summer]), April [early fall], May [middle fall], June [late fall]).
2.6. Fruit Sampling and Processing Procedures
Samples of Medfly-infested fruit were collected from all release sites 1 and 8 weeks after each release of D. longicaudata in order to monitor parasitoid recovery. Ripe fruits were collected from the ground below of the canopy of plants when available in backyard gardens and orchards. The fruit species surveyed were: Citrus reticulata Blanco (tangerine), C. sinensis (L.) Osbeck (sweet orange), C. paradisi Macfadyen (grapefruit) (Rutaceae), Diospyros kaki L. (persimmon) (Ebenaceae), Ficus carica L. (fig) (Moraceae), Malus domestica Borkh (Apple), Rosa canina (Bastard) Rapin (Rose) (Rosaceae), and Vitis vinifera L. (grape) (Vitaceae). Based on fruit weight, collected fruits were divided into the following categories each time fruits were sampled: small (1 - 8 g), medium (20 - 80 g), and large (100 - 250 g). Each medium and large-sized fruit sample contained 10 fruits while each small-sized fruit sample included 30 fruits. Three fruit samples per host plant species and per collecting date and site were taken to the laboratory. In the laboratory, each fruit was individually weighed and rinsed with a 30% solution of sodium benzoate. Fruit samples were placed in plastic crate (40 × 30 × 20 cm) containing poplar sawdust in the bottom as a pupation medium and it was tightly covered with an organdie cloth lid. Fruit samples were kept inside a dark room at 25˚C ± 1˚C, 65% ± 10% relative humidity during 4 weeks. Pupation medium was sifted weekly to collect Medfly pupae. Afterwards, fruits were dissected to determine the presence of Medfly larvae or pupae in the pulp. Live larvae were allowed to pupate and were then added to the other pupae collected from the same sample. All pupae were counted and transferred to plastic cups containing sterilized poplar sawdust as a pupation medium in the bottom and with an organdie cover in the top. Pupation medium was weekly moistened until all samples had been processed. Once a week, all emerged insects (parasitoids and flies) inside cups were counted and transferred into vials containing 70% alcohol.
2.7. Parasitoid and Fly Identification
Insects (parasitoids and flies) were identified by one of us (LS) using taxonomic keys [18] [19] .

Table 2. Releases of Diachasmimorpha longicaudata in San Juan, Argentina, during 2009.
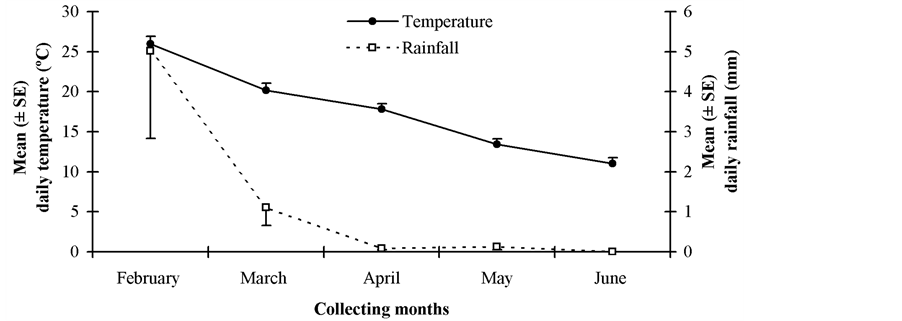
Figure 1. Mean (±SE) daily precipitation and temperature at the study region during parasitoid releasing months (February [middle summer], March [late summer]), April [early fall], May [middle fall], June [late fall]).
2.8. Data Analysis
Parasitism percentage was calculated as the total number of emerged parasitoid progeny divided by the total number of recovered Medfly pupae per 100. Even though this measure of parasitism underestimates the mortality inflicted by parasitoid females because some host larvae die after being stung [20] , it allows for comparisons between Medfly host fruits, and also as an indirect measure of parasitoid foraging preferences [21] . Fruit infestation values were based on the number of Medfy pupae per number of sampled fruit. The relationship between parasitism and larval infestation per fruit sample was analyzed by a Spearman Rank Order Correlation test (P < 0.05). Sex ratio of parasitoid offspring was calculated as the percentage of emerged females.
3. Results
Of the 144 fruit samples (=2280 fruit harvested), 2504 Medfly pupae were recovered. The Medfly infestation rate ranged from 6.1 to 0.1 pupae/fruit. Of total Medfly-infested fruit samples, 23 (16%) were parasitized by D. longicaudata. In total, 119 parasitoid adults were recovered from 6 Medfly-host fruit species which accounted for 75% of all plant species sampled. The highest number of parasitoids was recovered from fig, followed by grape, rose, orange, tangerine, and persimmon (Table 3).
Relative abundance of D. longicaudata by collecting month is presented in Figure 2. The highest abundance of parasitoids was recorded in summer. Over this season, 88% of the total number of adult parasitoids was recovered at the study areas.
The mean numbers of Medfly pupae and adults, parasitoid adults, parasitism percentage, and fruit infestation level recorded per collecting site, sample date, and fruit species are detailed in Table 4. Of the 6 sites where releases of D. longicaudata were performed, only in Ullum valley no parasitoids were recovered (Table 4).
The mean cumulative rate of parasitism by both fruit species and release site is shown in Figure 3. This rate of parasitism ranged from 13.4% in fig to 1.0% in Citrus spp. (tangerine and orange). Fruit weighing between 20 and 80 g (medium-sized fruit) were the most parasitized. Parasitism recorded from that fruit category was 18- and 3-fold higher than that obtained from largeand small-sized fruit, respectively (Figure 4). Overall, A strong positive correlation was found between parasitism and fruit infestation level (r = 0.73, N = 24, P < 0.0001).
4. Discussion
Diachasmimorpha longicaudata adults were successfully recovered from fruit samples belonging to 6 species of Medfly-host plants that were collected in 5 study sites a few weeks after beginning the releases. This would point out that the laboratory-reared parasitoids were in good condition upon release and were able to overcome local climatic conditions, to locate and parasitize Medfly larvae infesting different fruit species, and to develop

Table 3. Total number of D. longicaudata adults recovered from Medfly-infested fruit species collected in fruit-producing irrigated-valleys of San Juan, Argentina, through 2009.

Figure 2. Relative abundance of D. longicaudata per collection month at the study region.

Figure 3. Mean (±SE) cumulative rate of parasitism by D. longicaudata recorded from Medflyinfested fruit species at each release site. N = number of sampled fruit. Data were pooled by fruit species independent of sample date.

Figure 4. Mean (±SE) parasitism by D. longicaudata recorded from three Medfly-infested fruit categories based on individual fruit weight (small-, medium-, and large-sized fruit; see text for details). Data were pooled by fruit category independent of fruit species, sample date, and collection site.
at least one new generation in the field. No prior evidence of endemic parasitoid species associated with C. capitata has ever been found in San Juan. Only a cosmopolitan parasitoid species, Pachycrepoideus vindemiae (Rondani) (Pteromalidae) was circumstantially recorded from Medfly pupae [22] . This pteromalid species is a polyphagous idiobiont pupal parasitoid that is known primarily as parasitoid of synanthropic flies [5] .
Parasitoids recovered from small-, mediumand large-sized fruit that were collected from different release places indicated the wide range of host fruit accepted by D. longicaudata. Particularly medium-sized fruits were preferred by parasitoid females. This fruit category showed the greatest parasitoids abundance and the highest parasitism rate. This pattern was consistent with data recorded in the field in Veracruz, Mexico [11] [12] [23] and Florida, USA [21] , wherein D. longicaudata was predominantly common on mediumand large-sized fruits. The ability of laboratory-reared D. longicaudata females to attack C. capitata larvae infesting C. sinensis and C. reticulata in two release sites is particularly advantageous. This event has an important practical implication for the implementation of Medfly biological control in San Juan on two grounds. Firstly, Citrus species are among the most common and widespread Medfly host plants in the fruit-growing regions of Argentina and serve as

Table 4. Mean (±SE) numbers of C. capitata pupae and adults, D. longicaudata adults, parasitism, and infestation level recorded from host fruits collected in fruit-producing irrigated-valleys of San Juan, Argentina, through 2009.

Continued
aThree fruit samples per collecting date. bMean number recorded from 3 fruit samples; each sample was 10 medium or large fruits or 30 small fruits. cCc = Ceratitis capitata. dDl. = Diachasmimorpha longicaudata. eMedfly pupae/fruit.
important reservoirs for this exotic pest [24] . Secondly, it is known that tephritid fruit flies can escape parasitism if they infest large fruit, like Citrus spp., because the larvae can feed at a greater depth in the fruit pulp [11] [25] [26] . Therefore, mass releases of D. longicaudata in small citrus orchards, which are widely dispersed in the different irrigated fruit-producing valleys, could contribute to the suppression of Medfly populations. Recent field-cage studies have shown that D. longicaudata significantly contributed to C. capitata mortality on infested oranges and grapefruits [27] .
Interestingly enough, D. longicaudata adults were recovered from fruit samples collected from February to June, covering two seasons (summer and autumn). During collecting months mean daily maximum and minimum temperatures and rainfall varied from 24.8˚C to 18.3˚C, from 10.9˚C to 3.8˚C, and from 15.9 to 0.0 mm, respectively. This suggests an apparently good climatic adaptation by the parasitoid D. longicaudata in almost all release sites. Therefore, this raises our expectation for its establishment in the fruit-producing irrigated-valleys of San Juan. Consequently, a new fruit sampling only in order to monitor establishment in the parasitoid release places will begin on summer 2014-2015. It should be noted that permanent establishment of D. longicaudata was recently confirmed in the citrus-growing areas of the north-eastern and north-western regions of Argentina [28] [29] , where climate is characterized as subtropical temperate-warm humid. This exotic parasitoid species was recovered approximately 40 years after their first releases in northern Argentina. This is consistent with bioclimatic requirements by D. longicaudata because this exotic parasitoid species favors tropical and subtropical climates [7] . It is native to Southeast Asia and has been successfully established in tropical and subtropical regions of the world [5] [7] . However, its performance in an Argentinean fruit-producing area under temperate-cold dry climate, e.g. Cuyo region, was unknown until now. Even though several releases of D. longicaudata were performed under desert climate conditions of Israel from 1955 to 1959 and from 1966 to 1969 [30] , nobody has been able to recover it in more than 50 year [31] .
Even though it is yet too early to determine real effect of D. longicaudata on C. capitata populations, all of that preliminary information provided from this study opens up the possibility of delineating new area-wide control strategies of Medfly in San Juan. For example, the successful establishment of a mass rearing of D. longicaudata in the BioPlanta San Juan facility [1] allows its use in augmentative release programs during the maximum fruiting periods in the fruit-producing irrigated-valleys in semi-arid areas of San Juan. These parasitoid releases may be followed by mass releases of sterile Medflies and by the application selective toxic baits [8] [32] . Such actions will greatly contribute to the objectives of ProCEM-San Juan: establish both low C. capitata prevalence areas and free Medfly areas in fruit-growing valleys of San Juan based on the concept of area-wide integrated pest management [1] . Nevertheless, studies on the bioclimatic requirements of D. longicaudata as well as assessment of parasitoid efficacy through post mass release monitoring are still needed in these fruit-producing semi-arid areas.
5. Conclusion
The findings from this study provide clear evidence that D. longicaudata may contributed to Medfly mortality in different host fruit species growing in the fruit-producing irrigated-valleys of San Juan.
Acknowledgments
We are thankful to Mariana Bilbao, Rita Rosselot (ProCEM-San Juan), Carolina Chiappini, Josefina Buonocore, Lorena Escobar, Patricia Albornoz-Medina, Natalia Salinas, Patricia Colombres, Ulises Chaya, Guillermo Borchia, and Liliana Colombres (PROIMI-CONICET) for valuable technical assistance. Financial support was provided by the San Juan Eradication and Control Medfly Program (ProCEM San Juan, Argentina), and by the Agencia Nacional de Promoción Científica y Tecnológica de Argentina (ANPCyT) through Fondo Nacional de Ciencia y Tecnología (FONCyT) (grants PICT/2006 No. 2402 and PICT/2010 No. 0393).
NOTES
*Corresponding author.