Synthesis and Characterisation of a Biolubricant from Cameroon Palm Kernel Seed Oil Using a Locally Produced Base Catalyst from Plantain Peelings ()
1. Introduction
The world’s crude oil reserves are rapidly depleting as a result of high consumption which exceeds the rate of natural formation of mineral deposits [1] and the world runs the risk of a severe energy crisis if rapid alternative energy solutions are not sought [2] [3]. These impending energy crises have provoked scientific and technological research on bio-based materials as alternative energy sources. The long term solutions to our energy needs depend on the development of renewable, biodegradable, and environmentally friendly industrial products such as biodiesel, biolubricants and other fuels that potentially substitute or subsidize conventional fuels and reduce the dependence on fossil fuels [4] [5] [6] [7] [8].
Lubricants minimize frictional resistance between surfaces in relative motion. They reduce mechanical wear and tear of machine components thereby increasing their life span and efficiency and also facilitate heat transfer, liquid sealing, contaminant suspension, and corrosion protection [1] [9]. There are two main types of lubricants namely, lubricants obtainable from fossil sources and those produced from vegetable oil sources. The demand for biodegradable and environmentally friendly bio-lubricants is on the increase, hence the need for scientific and technological research into the synthesis and production of such bio-lubricants from available vegetable oil sources. Similar work in this area includes the production of biolubricants from Nigerian Jatropha curcas seed oil [1]. Jatropha oil is not edible but highly unsaturated with attendant thermal instability. Consequently, biolubricant synthesis from Jatropha is a labor and capital intensive process.
We report here the synthesis and characterization of a bio-lubricant from Cameroonian palm kernel seed oil obtained from agricultural and industrial waste, emanating from palm oil processing industry in Cameroon. The oil is not edible and some is used as a raw material for the production of soaps and detergents.
2. Materials and Methods
2.1. Materials
Palm kernel seeds (Elaeis guineensis) were collected from the dumping site at an artisanal oil mill in Widikum (Cameroon) and mechanically cracked to separate the seeds from the hard shells. The seeds were sun-dried for 3 days to reduce the moisture content. The dried palm kernel seeds were used to extract palm kernel oil by mechanical press and solvent extraction. The palm kernel oil was and then characterized by physical and chemical methods. Reagent grade methanol, potassium hydroxide (KOH), ethanol, ether, chloroform were used as purchased without further purification.
2.2. Palm Kernel Oil Extraction
Two kilograms (2 kg) of palm kernel seeds were crushed to a particle size of about 3 mm to allow solvent penetration and oil percolation and heated to about 80˚C with open steam thereby humidifying the materials and raising the moisture content. The material was then flaked, and conveyed to the mechanical extracting system. For solvent extraction, 100 g of crushed palm kernel seeds was placed in the thimble-holder in a 2-L capacity Soxhlet extractor and diethyl ether (300 mL) added into the distillation flask [10]. The heating of the Soxhlet was regulated so that it made 4 siphons in 1 hour for a total of 7 hours for complete extraction to be achieved. The heating mantle was switched off and the extract allowed to cool down. The mixture was then gently heated to evaporate diethyl ether leaving palm kernel oil. The extracted PKO was characterized and the results are published [11].
2.3. Biolubricant Synthesis by Double Transesterification of Palm Kernel Oil
The PKO biolubricant was synthesized using the double transesterification method [1]. The first step produced intermediate products of methyl esters of fatty acids which were used in the second step to produce the biolubricant in the presence of trimethylolpropane (TMP). The two processes were carried out as described below.
2.3.1. Production of KOH Using Plantain Peelings
An alternative source of the base catalyst was explored primarily for the sake of economics of production. The catalyst precursor was derived from plantain fruit (Musa paradisiaca) peelings, an urban waste product. Three kilograms of plantains peelings were collected from an urban waste dump in the Bamenda (Cameroon) municipality, washed with water to remove soil, debris and other impurities and sun-dried for one week to reduce the moisture content and to render the peelings easily combustible [12] [13]. The plantain peelings were then burnt in an air-rich metallic container; the ash was collected and stored in a labeled polythene bag. The residual ash is predominantly potassium oxide (K2O) which dissolves in water to give KOH solution. The base catalyst (KOH) was extracted from the ash following a modified procedure by Enontiemonria et al. [13]. In this extraction, 30 g of dry ash was dissolved in 300 mL of mineral free water using 500-mL beakers. The beaker and its content were placed on a hot plate magnetic stirrer and kept at 50˚C for 4 hours. The resultant solution was then filtered using filter paper and stored. The filtrate was collected in a stainless steel container, verified with litmus paper for pH value and evaporated to dryness. The dried residue was weighed and stored in a plastic container. The percentage of KOH recovered was calculated using Equation (1).
(1)
2.3.2. Synthesis of Methyl Ester using Conventional KOH
Purified PKO (100 mL) was transferred into a heating pan and warmed to 50˚C. Methanol (24 mL) was transferred into a beaker and potassium hydroxide (KOH) (0.9234 g) added and stirred magnetically until all the KOH was completely dissolved. The warm PKO was transferred into the reaction vessel (500-mL round bottom distillation flask) and the catalyst (1% mass of PKO) was added. The contents of the reaction vessel were swirled vigorously for five minutes. The reaction mixture was then heated under reflux in a water bath at 70oC for 45 minutes during which the transesterification reaction took place. The content of the reaction vessel was transferred into a separatory funnel and visible separation of methyl esters as the supernatant liquid was observed after 15 minutes. Overnight, the reaction mixture separated into the upper amber colored methyl esters layer and the lower impure glycerol layer (see Figure 1). The products were separated by allowing each fraction to flow into separate labeled measuring cylinders. The methyl esters were purified using the modified procedure by Aladetuyi et al. [14] and the yield of methyl ester calculated using Equation (2) [14] [15] [16].
(2)
where Vp is the volume of product and Vs the volume of sample oil used for the synthesis.
2.3.3. Synthesis of Biolubricant
The synthesis of biolubricant from palm kernel oil methyl esters (PKOME) by transesterification with trimethylolpropane (TMP) is depicted by the following stoichiometric equation (Scheme 1).
![]()
Figure 1. Methyl esters synthesized (Upper layer).
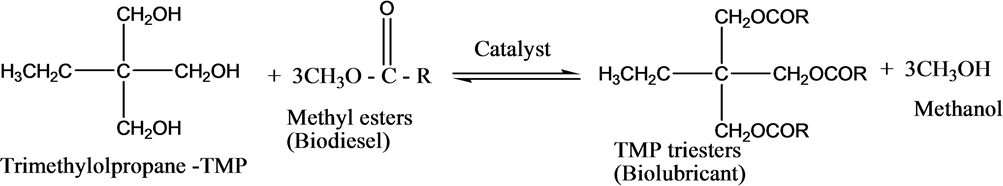
Scheme 1. Production of TMP Biolubricant.
The previously prepared palm kernel oil methyl esters (PKOME) (100 mL) in a 500-mL reaction vessel was heated in a water bath to 70˚C and 0.9 g of KOH base catalyst solution was added. After 10 minutes, 20 g of TMP crystals were added to the reaction vessel and the reaction allowed to proceed for 4 hours at 100˚C under reflux. The reaction mixture allowed to cool to room temperature. The mixture was transferred into a separatory funnel and the TMP triester (biolubricant) was collected as the bottom viscous layer. The biolubricant yield was calculated using Equation (3) [17].
(3)
where Vp is the volume of product and Vs the volume of sample taken.
2.3.4. Biolubricant Synthesis Using KOH from Plantain Peelings
Biolubricant was synthesized using locally made base-catalyst. 100-mL of palm kernel oil methyl esters (PKOME) was measured into a 500-mL reaction vessel. 4.62 g of locally made KOH corresponding to 5% weight of PKOME was dissolved in 10 mL of methanol with the aid of a magnetic stirrer. The catalyst solution was added to the 500-mL reaction vessel and the mixture swirled for five minutes followed by the addition of 20 g of TMP to the reaction mixture which was then maintained in a water bath at 100˚C for 4 hours. The reaction mixture was allowed to cool to room temperature. The mixture was transferred into a separatory funnel for the biolubricant to decant as the bottom viscous layer which was collected directly into a measuring cylinder. The supernatant layer was recycled by transferring it back to the reaction vessel and adding more catalyst and TMP and repeating the heating process for four hours. The lubricant formed in the recycled batch was separated and the total yield computed.
2.4. Characterization of PKO Biolubricant
The biolubricant was analyzed for its physical and chemical properties [18] [19]. The specific gravity was determined using the density bottle method while the rest of the properties were determined using standard analytical methods as shown in Table 1 [1] [20].
A sample of commercial petroleum lubricant was obtained from the Quality Control Laboratory of the Kaduna Refinery and Petrochemical Company (KRPC) and subjected to similar analyses to compare with synthesized biolubricant from palm kernel oil. The chemical compositions of both lubricants were investigated using the Gas Chromatography-Mass Spectrometry QP2010 plus with helium gas as the carrier medium.
3. Results and Discussions
The physico-chemical properties of the products are shown in Table 2. Bio-based lubricants derived from vegetable oils are biodegradable and have lower eco-toxicity compared to petroleum lubricants [21]. However, several studies
![]()
Table 1. Characterization of PKO Biolubricants by Standard Analytical Methods [20].
![]()
Table 2. Characteristics of PKO, Biolubricants and Petroleum Lubricant.
have shown that vegetable oils have limitations as lubricants without structural modification [21] [22] [23]. Such limitations include thermal, oxidative and hydrolytic instability and inadequate low temperature fluidity due to high pour points [21] [24]. These limitations are due to the presence of the glycerol moiety which is a major component in vegetable oil. The susceptibility of this molecule to high temperature destruction is due to the presence of a β-hydrogen atom on the second carbon atom of the glycerol molecule [22] [23] which is more acidic and unstable. Most of these shortcomings can be minimized by chemical modification through transesterification of vegetable oil with polyhydric alcohols [21].
The PKO biolubricant synthetic method (double transesterification) causes the elimination of the β-hydrogen atom from the vegetable oil structure and provides an ester with high degree of oxidative and thermal stability which is seldom found in vegetable oils [25]. In the double transesterification procedure, the glycerol molecule bearing the unstable hydrogen in vegetable oil is substituted by a more stable TMP. The first stage of transesterification gets rid of the glycerol molecule while the second stage puts a more potent molecule (TMP) in the place of glycerol thus giving trimethylolpropane triester (TMPTE) which has superior properties and performance.
The yield of biolubricant calculated from conventional base catalyst was 51.0% while that from locally produced base catalyst was 48.6%. This implies that the locally produced catalyst is almost as effective as the conventional, imported catalyst. This minimizes production cost, thus reducing the cost of the biolubricant and enhancing affordability and valorizing municipal waste through transformation into a useful product for the conversion of industrial wastes (palm kernel seeds) into biolubricant. This is in conformity with the observation that energy conversion is sustainable and cheaper when it is derived from waste and renewable biomass [26].
In order to evaluate the propinquity of the synthesized biolubricant to the conventional lubricant, a petroleum lubricant sample was also analyzed using similar parameters as those determined for the biolubricant. The results in Table 2 show that biolubricant is heavier and more viscous than the petroleum lubricant. These properties are responsible for the higher mechanical (load) and thermal resistance of the biolubricant over the mineral lubricant. The viscosity indices and cold flow properties are generally similar indicating that the biolubricant can be blended with the petroleum counterpart to complement one another and thus produce a hybrid and more synergistic product. The propensity to fire hazards of products is determined by the flash and fire points. The flash and fire points of the biolubricant are slightly greater than those of the vegetable oil PKO showing the enhanced thermal resistance of the product of transesterification with TMP. The flash and fire points of the biolubricant are not too different from petroleum lubricant implying its greater thermal stability. The biolubricant may therefore require some antifoaming additives to complement its properties as it exhibited slight foaming at higher temperatures.
3.1. Variation of Specific Gravities (SG) of Products
From the results obtained, the specific gravity of PKO biolubricant catalyzed by locally produced base catalyst, 1.22 g/mL is similar that of the PKO biolubricant catalyzed by conventional base catalyst, 1.20 g/mL. Both SGs are higher than that of precursor PKO, 0.923 g/mL; with the petroleum lubricant having the lowest specific gravity value at 0.848 g/mL. The specific gravity value is lower for palm kernel seed oil than for biolubricant due to an increase in molecular complexity brought in by the trimethylolpropane (TMP) backbone. The specific gravity value for the biolubricant is higher than that of the petrolubricant due to a change in chemical structure of constituent molecules which are predominantly saturated aliphatic and few aromatic hydrocarbons. The specific gravity of the biolubricant catalyzed by locally produced base catalyst is higher probably due to slightly higher impurity level as suggested by the ASTM color values. The change in specific gravity leads to a corresponding change in the mass of the products. The greater the specific gravity, the heavier and more viscous the oil is, and such a lubricant does not easily thin out at higher temperature and can withstand greater loads. The specific gravity is also an indicator of product adulteration. The SG of the biolubricant for PKO, biolubricant and petrolubricant determines the compatibility of the products with either the heavy or light duty engines which is the ability of a sample to mix with other liquids [27]. Substances with smaller SG (<1) can float in water while those with SG greater than one sink in water. The persistence of biolubricant on applied surfaces and joints would be longer due to higher specific gravity and consequently, higher viscosity.
3.2. Kinematic Viscosities of Products at 40˚C and at 100˚C
The viscosities of biolubricants were 509.80 cSt at 40˚C and 30.80 cSt at 100˚C respectively using conventional base catalyst (KOH). The values for the biolubricant catalyzed by base catalyst produced from plantain peelings were comparatively high as well. These values for PKO biolubricant are greater than those of biolubricant from Jatropha oil which were reported as 55.17 cSt at 40˚C and 10.96 cSt at 100˚C respectively [1]. The values for PKO biolubricant are higher than those of petroleum lubricants which were measured as 46.476 cSt at 40˚C and 6.9404 cSt at 100˚C. This is indicative that PKO biolubricant possesses greater thermal stability and can withstand greater mechanical stress than petroleum lubricants. Viscosity is a measure of the ease of flow of a fluid. It is determined by measuring the amount of time taken for a given measure of fluid to flow through an orifice of a specified size [28]. The viscosities of products at 40˚C are generally much greater than the viscosities at 100˚C due to the fact that intermolecular forces resisting flow in liquids such as hydrogen bonds and van der Waals forces are largely broken down at higher temperatures.
3.3. Viscosity Index Variation
The viscosity index values for the PKO biolubricants catalyzed by conventional and unconventional catalysts and petro-lubricant are 90, 110 and 105 respectively. The values are moderately high and very close to that of the petroleum lubricant. Hence chemical synthesis has produced a lubricant from organic and renewable sources with similar properties to lubricants of mineral origin more especially with the added advantage of biodegradability. Most liquids tend to thin out when temperatures increase and thicken when temperatures decrease. The property of a liquid to resist changes in viscosity when temperature increases or decreases is called viscosity index. Higher values of viscosity index are desirable because such a lubricant greatly resists changes in viscosity when temperature changes. The viscosity index of PKO is high (185) in addition to its high flash point of 200˚C. This implies, pure palm kernel oil itself is a potent lubricant with some limitations when used at high temperatures [27]. The high temperature limitations stem from the glycerol backbone in triglycerides which are significant chemical components of vegetable oils. This glycerol component is readily destructible at high temperatures due to the presence of hydrogen atoms in the beta position relative to the hydroxyl group in the glycerol molecule [21].
3.4. Cold Flow Properties
The cloud point of oil refers to the temperature at which wax first become visible as the temperature is lowered while the pour point is the temperature at which the oil solidifies enough to resist flow [29]. The cloud point was reduced from 29˚C for PKO to 0˚C for biolubricants after double transesterification and the process similarly reduced the pour point of palm kernel oil from 20˚C to 0˚C in biolubricants. The clouds points of the biolubricants are 0˚C each while the pour points are −8 and −9˚C. The cloud point of the petroleum lubricant is −5˚C while the pour point is −20˚C. These results suggest that the cold flow properties of the biolubricants and petro-lubricant are adequate to permit their use at extreme temperatures. For the biolubricant, the thermal resistance of the product is enhanced as the thermally fragile glycerol in the palm kernel oil triglycerides is replaced by the trimethylolpropane backbone which is thermally stable. Hence the biolubricant synthesized from palm kernel oil has better properties in terms of thermal stability and cold flow, compared to neat palm kernel oil lubricant.
3.5. Flash Points of Products
The flash point of a fuel refers to the temperature at which the fuel can ignite when exposed to a heat source. The flash point is of relevance when safe handling, storage and transportation are concerned [30] [31]. The flash points of PKO and PKO biolubricant were 200˚C and 210˚C respectively. These products are therefore classified as non-hazardous products because of their high flash points. The flash point of a lubricant refers to the temperature at which some vapor is emitted from the substance to momentarily ignite a flame while the fire point is the temperature at which enough vapor is emitted from the substance undergoing a heating program to sustain a flame [32]. The flash and fire points are the properties that must be considered in assessing the overall flammability hazard of a biolubricant and related materials. The neat flash point and fire points of PKO biolubricant were not obtained with certainty because the product foamed at 210˚C. The flash and fire points of the petroleum lubricant were 220 and 231˚C respectively. The approximate results are in the range of those reported by Mohammed et al. [23] which gave the flash point of Neem biolubricant as 262˚C and that of Jatropha biolubricant as 274˚C [1]. The PKO biolubricant thus exhibited some foaming characteristics. Foaming characteristics in an industrial lubricant is undesirable as it may interfere with the overall system performance and may lead to some mechanical damage. Consequently, antifoaming agents may be used to minimize the foaming propensity of the PKO biolubricants synthesized.
3.6. IR Analysis of Biolubricant
Vibrational frequencies of organic molecules are widely used in qualitative and
![]()
Figure 2. IR Spectrum of PKO Biolubricant.
quantitative analysis to identify different absorption chromorphores. The vibrational frequencies are sub-classified into group frequencies characteristic of groups of atoms or functional groups and fingerprint frequencies characteristic of specific molecules. The synthesized biolubricant was analyzed by FTIR to ascertain that the transesterification reaction between methyl esters and trimethylolpropane (
TMP
) did effectively take place. The IR spectra of the PKO biolubricant are elucidated by Figure 2.
From the spectrum, the prime peak of relevance occurs at 1743.7 cm−1 which falls in the range of carbonyl (C=O) absorption for esters [33]. The absorption peaks at 2931 and 2886 cm−1 fall in the absorption range for C-H stretching in the hydrocarbon component of the biolubricant. The broad absorption peak at 3340 cm−1 suggests the presence of O-H groups from some
TMP
impurity molecules.
4. Conclusions
A biolubricant with good lubricant properties for higher temperature applications has been produced from Cameroon palm kernel oil. A new base catalyst was produced from municipal wastes (plantain peelings), whose performance was very close to that of conventional base catalyst (KOH) used in this work. We have therefore used local materials to produce a biolubricant using cheap base catalyst.
However, further research is required to improve on the yield of the biolubricant, optimize the production of the base catalyst from plantain peelings and improve the purity level. Also the performance of the synthesized biolubricant needs to be studied in a sample engine to evaluate its suitability in various engines.
Conflicts of Interest
The authors declare no conflicts of interest regarding the publication of this paper.