1. Introduction
Polyurethane (PU) has been widely used in a variety of applications in many different fields. In general, PU suffers from heat resistance. However, by improving this fundamental property, PU may gain some industrial advantages. Therefore, chemists have actively pursued PU heat resistance. Polyurethane-imide (PUI), which has stability in high-temperature environments, excellent electrical and mechanical properties, and good chemical resistance, can be prepared via an organic-organic hybrid between urethanes and imides [1] - [18] . PUI possesses some distinct properties such as heat resistance and solvent resistance. Therefore, PUI has found special applications in selected industries and continues to gain importance in a variety of applications. PUI is used in a surprising number of commercial applications. For convenience, PUI is classified into three major types of products: foams [19] [20] [21] [22] , elastomers [23] - [30] , and resins [5] [31] [32] [33] . However, a particularly interesting material is polyurethane-imide elastomer (PUIE). We focused on PUIE because it is a novel heat-resistant elastomer that is widely used for commercial products, and we were successful in improving the synthetic method for creating PUIEs [4] [9] [15] . Moreover, we focused on thermoplastic PUIEs (TPUIEs) that could be utilized as industrial materials. Some of the most important TPUIE attributes include their ability to be reprocessed, the wide range of colors obtainable, and their worldwide availability. Although TPUIEs are industrially utilized, they are often reported on in the field of PUIEs. From both the theoretical and practical standpoints, it is important to synthesize TPUIEs that maintain their thermoplasticity. This becomes a key challenge in the field of polymer chemistry, and it is important that an expression of PUIE thermoplasticity is clarified.
In this article, we used the following materials for the synthesis of new PUIEs: 4,4'-diphenylmethane diisocyanate, polyols (polytetramethylene glycol (Mw = 1000), polycaprolactone (Mw = 1000), and polycarbonate diol (Mw = 1000)), 4,4'-oxydianiline, and acid anhydrides (pyromellitic dianhydride and 4,4'-oxydiphthalic anhydride). The resulting thermoplasticities were examined.
2. Experimental
2.1. Materials
4,4'-Diphenylmethane diisocyanate (MDI) and polyols (polytetramethylene glycol (Mw = 1000) (PTMG1000), polycaprolactone diol (Mw = 1000) (PCL1000), and polycarbonate diol (Mw = 1000) (PCD1000)) were supplied by Tosoh Industry, and they were purified by distillation or dried prior to use. Pyromellitic dianhydride (PMDA), 4,4'-oxydiphtaric anhydride (ODPA), and 4,4'-oxydianiline (ODA) were purchased from Tokyo Chemical Industry. N-Methyl-2-pyrrolidone (NMP) was purchased from Nacalai Tesque, Inc., purified by distillation, and stored over 4Å molecular sieves.
2.2. Synthesis
Scheme 1 shows our synthetic method for preparing the PUIEs. Table 1 summarizes the composition of the PUIE composites. All the PUIEs were prepared by liquid polymerization. PUIEs containing 35% polyimide were synthesized. MDI and polyols (PTMG1000, PCL1000, and PCD1000) were placed in a 100 mL four-necked separable reaction flask equipped with a mechanical stirrer, a gas inlet tube, and a reflux condenser, and the mixture was stirred at 80˚C for 30 min. To this reaction mixture was added PMDA and NMP (15 mL), and the mixed solution was stirred at 150˚C for 15 min. ODA in NMP (10 mL) was added, and the mixture was then stirred at 150˚C for another 2 h. The reaction was carried out under an Ar atmosphere. A test film was next prepared from the synthesized PUIEs by casting half of the obtained polymer solution using a centrifugal casting machine at 150˚C overnight, after which the film was treated at 200˚C for 4 h in vacuo (267 - 400 Pa).
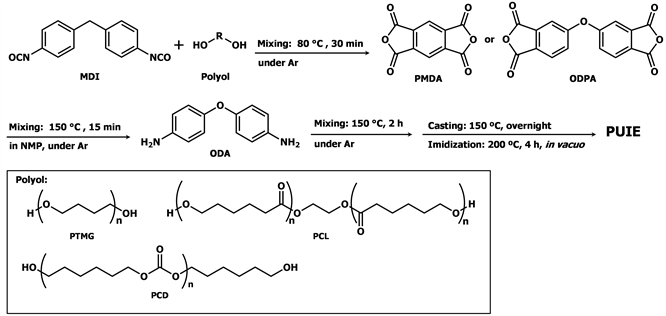
Scheme 1. Solution-phase synthesis of PUIEs.
a4,4'-MDI: Mw = 250.25; bPTMG1000: Mw = 1000; PCL1000: Mw = 1000; PCD1000: Mw =1000; cODA: Mw = 200.24; dPMDA: Mw = 218.12.
2.3. Characterization
2.3.1. Chemical Properties
Swelling tests were carried out using 0.1000g test pieces. The degree of swelling (Rs) was calculated using the formula Rs (%) = (W' − W)/W × 100, where W' is the weight of the test piece soaked in benzene for 24 h, and W is the weight of the test piece after drying at 30˚C for 24 h in vacuo.
2.3.2. Mechanical Properties
Tensile tests were performed on an OrientecRTC-1225A (Tokyo, Japan) with a model-U-4300 using a JIS 3-dumbbell as the standard sample and a crosshead speed of 100 mm/min.
2.3.3. Thermal Properties
Differential scanning calorimetry (DSC) measurements were performed on a Rigaku Thermo-Plus DSC-8230 (Tokyo, Japan) at 10˚C/min from −120 to 200˚C under an Ar atmosphere. Approximately 9.5 mg of each PUIE was weighed and sealed in an aluminum pan. The samples were rapidly cooled to −120˚C and then heated to 200˚C at 10˚C/min.
Dynamic mechanical analysis (DMA) was performed on a Seiko Instruments DMS 6100 (Chiba, Japan) at 5˚C/min over −100˚C to 300˚C at 20 Hz under a N2 atmosphere.
Thermogravimetric analysis (TGA) was performed on a Seiko Instruments TG/DTA6200 device (Chiba, Japan) at a heating rate of 10˚C/min from 30 to 500˚C under a N2 atmosphere.
2.3.4. Molecular Calculations (Gaussian)
Molecular calculations were carried out using the Gaussian09W program at the B3LYP/6-31G(d) level. The resulting molecular models were visualized using Gauss View 5.0.
3. Results and Discussion
Expression of Thermoplasticity
The definition of a thermoplastic elastomer in JIS appears to be the following: “A polymer, or blended polymer, showing a vulcanized rubber-like nature at the operating temperature that can be processed and reprocessed to recover the polymer, or blended polymer, at high temperature.” Currently, there is no defined test method for evaluating thermoplasticity. Therefore, we defined thermoplasticity as follows: “An elastomer showing elasticity at the operating temperature that can be reprocessed at higher temperatures. Moreover, the nature of the reformed elastomer must demonstrate the same properties as the original material.”
Synthesis of the PUIEs was carried out using various raw materials and the PUIE films fused during the process of imidization. A heating test was carried out on the PUIE films (Figure 1). The PUIE films that contained ODPA became fused, whereas the PMDA-containing PUIEs burned and carbonized. This suggests that thermoplasticity only appears in the PUIEs that contained ODPA as the acid anhydride. To confirm the thermoplasticity of PUIEs synthesized with ODPA, a press molding was carried out. Then, tests for the chemical and physical properties (swelling test, tensile test, DSC, DMA, and TGA) of the PUIEs before and after the molding were carried out.
The swelling rate and Tg values of PUIEs both before and after the molding were nearly identical (Table 2). Figures 2-4 show the stress-strain curves, tanδ, and T5 and 50, respectively. The stress-strain curves, tanδ, and T5 and 50 of the PUIEs both before and after the molding were also identical.
Table 3 shows that the PUIEs expressed thermoplasticity. It has been suggested that the structure of ODPA in the PUIEs is related to the thermoplasticity. Therefore, to clarify correlation between the thermoplasticity and the structure of the acid anhydride raw material, the molecular orbitals of the PUIEs were calculated using the Gaussian program. ODPA, PMDA, ODPA-containing PUIE, and PMDA-containing PUIE were used as the model chemical compounds. Figure 5 and Figure 6 show the calculated results. The flexibility of the PMDA molecule, which has an aromatic ring and an imide group in the same plane, is relatively low and the molecule is fairly restrained. In contrast, the flexibility of the ODPA molecule, which has an oxygen atom between the two
![]()
Figure 1. Heating test of PUIEs: PUIE-ODPA and PUIE-PMDA.
aMeasurement conditions: benzene solvent at room temperature (23˚C ± 2˚C) for 24 h; bDSC was performed at a heating rate of 10 ˚C/min from −100˚C to 300˚C under an Ar atmosphere; cTGA was performed at a heating rate of 10 ˚C /min from 30˚C to 500˚C under a N2 atmosphere.
![]()
Figure 2. Tensile tests (stress-strain curves) of the PUIEs: solid line (before PUIE-ODPA) and dotted line (after PUIE-ODPA).
aromatic rings, is relatively high and the molecule is not restrained (Figure 5). Moreover, the flexibility of the PUIE is restrained by the π-π interactions between the diamine (ODA) and the aromatic ring. The expression of thermoplasticity for the ODPA-containing PUIE is thought to be related to the flexibility of the PUIE molecule, and this flexibility results from the molecular structure of ODPA (Figure 5). This concept of thermoplasticity is supported by the swelling tests and the DMA results as well.
![]()
Figure 3. Storage modulus and tan δ of the PUIEs as a function of temperature: solid line (before PUIE-ODPA) and dotted line (after PUIE-ODPA).
![]()
Figure 4. TGA curves of the PUIEs: solid line (before PUIE-ODPA) and dotted line (after PUIE-ODPA).
![]()
Table 3. Expression of the thermoplasticity of PUIEs.
a○: Thermoplasticity, ×: No Thermopasticity.
![]()
Figure 5. Molecular models of ODPA and PMDA.
![]()
Figure 6. Molecular models of ODPA-containing PUIE and PMDA-containing PUIE.
4. Conclusion
The expression of PUIE thermoplasticity for the various PUIEs synthesized by solution-phase polymerization was investigated. The thermoplasticity of ODPA-containing PUIEs was identified by the heating tests, swelling tests, tensile tests, DSC, DMA, and TGA of the PUIEs both before and after a molding treatment. We found that using ODPA as the acid anhydride greatly increased the thermoplasticity of the synthesized PUIE. The knowledge obtained in this study will be useful for designing TPUIEs for various applications.
Acknowledgements
The authors are grateful to Mr. Tomohiro Nishio for taking measurements using dynamic mechanical analysis.