1. Introduction
To address the issue of development of synthetic procedures which are environmentally more friendly, chemists have established different approaches and techniques [1] . Novel synthetic methods are focused on the economy of process by the reduction of waste production, either by application of more efficient catalysts, or procedures. The reduction of energy consumption during the synthesis also decreases impact on the environment, for instance more efficient heat trans- fer was achieved by employment of microwaves. Photochemistry employs light as a renewable energy source as a method for energy input. One of the methods for minimization of impact on the environment is the replacement of toxic reactants with less toxic. The replacement of solvents by less harmful, and ultimately water as the most benign, and conduction of reactions in solvent-free conditions would be the ultimate goal. Organic reactions could be effectively carried out without the presence of solvent in a microwave reactor, or simply by grinding reactants using mortar and pestle. Manual grinding is often associated with low reproducibility of results, and automated grinding in ball milling machines is gradually gaining in importance. Recent literature demonstrates the utility of mechanosynthesis in many common organic reactions [2] . This method is often adventitious over classical synthesis in solution in terms of reaction yield, reaction time, simplicity of workup, use of energy and solvents. In our ongoing research program on development of eco-friendly synthetic protocols [3] , we investigated the applicability of mechanosynthesis in deprotection of Boc-protec- ted amines, as a widely used protection group. As far as we are aware, ball milling technique was only used in amine Boc protection with di-tert-butyl dicarbonate [4] and synthetic methods for solvent-free removal of Boc group were reported in several papers. These include grinding in a mortar with iodine [5] , thermolysis at 180˚C - 185˚C [6] , or by thermolysis on silica gel at mild temperature (50˚C) at reduced pressure [7] [8] . Microwave-assisted thermolytic deprotection was achieved on silicagel within 1 min [9] or by MW heating on neutral alumina with AlCl3 in 1 min [10] . For deprotecion at low temperatures (rt to 40˚C), Lewis acid, YbTf3 was added on silicagel [11] . The objective of this account was to study the viability of solvent-free mechanochemical N-Boc deprotection reaction. Novel mechanochemical methodology could find its application in different areas of organic chemistry and complement existing synthetic methods.
2. Results and Discussion
When substrate 1 was ball milled with catalytic amount of iodine, or with silicagel for 60 minutes at room temperature, deprotection was not observed, which is in variance to our assumptions based on the published results. In principle, transfer from manual to automated grinding should facilitate reaction, and mild heat generated in ball milling vessel should be cooperative in deprotection by neat grinding. We have found that the mechanochemical removal of N-Boc group was effected by the solvent-free ball milling of substrate 1 with an excess of p-toluenesulfonic acid (Scheme 1), which is a modification of the procedure employing MW heating of substrates with p-TsOH [12] .
Mechanochemical removal of Boc group by p-TsOH was applied to substrates 1-5 and the corresponding tosylate salts were obtained in almost quantitative yields in short reaction time of 10 min at room temperature (Table 1). Reaction progress followed by TLC and NMR spectra of crude reaction mixtures indicate full conversion to products. Simple workup procedure consists of precipitation of products from suspension in dichloromethane. Inspection of results presented in Table 1 reveals that these mild reaction conditions are suitable for chemoselective deprotection of Boc-protected primary amines and do not affect cleavage of other amide or ester groups. In addition to simple alkyl amines, we have shown that this method is applicable to amino acids and their derivatives.
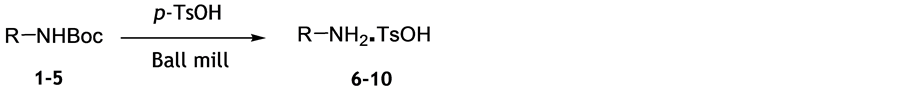
Scheme 1. Deprotection of N-Boc group by ball milling.
![]()
Table 1. Deprotection of N-Boc group in ball milla.
a10 min; bisolated yields, calculated on the basis of the moles of substrate.
3. Conclusion
A novel, simple solvent-free synthetic procedure for N-Boc removal in ball mill in very mild reaction conditions at room temperature is described. This method has potential application in Boc group deprotection of other types of amines, in various fields such as synthesis of pharmaceutically interesting molecules, natural product synthesis, or organic material science.
4. Experimental
General. Retsch MM400 mixer mill was used for ball milling experiments at frequency of 30 Hz, with stainless steel vessel (10 mL) and one stainless steel ball (10 mm). Solution 1H NMR spectra were acquired on Brucker Avance (300 and 600 MHz) spectrometers in deuterated DMSO with tetramethylsilane as an internal standard. FTIR-ATR spectra were recorded using a Fourier Transform-Infrared Attenuated Total Reflection PerkinElmer UATR Two spectrometer in the range 400 cm?1 to 4000 cm?1.
General procedure for Boc deprotection. A mixture of Boc-protected amine and 2 equivalents of p-toluenesulfonic acid monohydrate per Boc group was ground neat using 10 mm stainless steel ball at 30 Hz for 10 minutes at room temperature. The crude mixture was suspended in dichloromethane and the precipitate was collected by filtration. The resulting solid was air-dried to give deprotected amine as toluenesulfonic salt. In the case of 1, the crude mixture was soluble in dichloromethane, so the solution was evaporated to give the final product. Purity of all p-toluenesulfonate salts is evidenced by NMR spectroscopy, IR spectrometry and thin layer chromatography.
N-aminopropyl-N'-(L-phenylalanine methyl ester)oxalamide p-toluene- sulfonate (6)
White solid (quant. yield) 1H-NMR (DMSO-d6): δ (ppm) 1.65 - 1.77 (m, 2H, CH2), 2.29 (s, 6H, CH3-tol), 2.69 - 2.79 (m, 4H, CH2), 3.11-3.21 (m, 4H, CH2), 3.65 (s, 3H, CH3), 4.54 - 4.64 (m, 1H, CH), 7.14 (d, 4H, J = 7.9 Hz, Ar-tol), 7.19 - 7.31 (m, 5H, ArH), 7.49 (d, 4H, J = 7.9 Hz, Ar-tol), 7.67 (brs 3H, NH3+), 8.87 (t, 1H, J = 6.3 Hz, NH), 8.96 (d, 1H, J = 8.7 Hz, NH). IR (ATR) νmax/cm−1 3354, 3307, 3059, 2965, 1741, 1681, 1658, 1507, 1446, 1244, 1169, 1122, 1033, 1005, 855, 815, 745, 701, 683, 562, 529.
1,3-diamino p-toluenesulfonate (7)
White solid (quant. yield) 1H-NMR (DMSO-d6): δ (ppm) 1.78 - 1.91 (s, 2H, CH2), 2.29 (s, 6H, CH3-tol), 2.81-2.94 (m, 4H, CH2), 7.14 (d, 4H, J = 7.9 Hz, Ar-tol), 7.51 (d, 4H, J = 7.9 Hz, Ar-tol), 7.79 (brs 6H, NH3+). IR (ATR) νmax/cm−1 3061, 2970, 1710, 1598, 1526, 1494, 1390, 1188, 1118, 1028, 999, 814, 678, 558.
1,5-diaminopentane p-toluenesulfonate (8)
White solid (quant. yield) 1H-NMR (DMSO-d6): δ (ppm) 1.26 - 1.38 (m, 2H, CH2), 1.46 - 1.59 (s, 4H, CH2), 2.29 (s, 6H, CH3-tol), 2.76 (t, 4H, J = 7.7 Hz, NCH2), 7.13 (d, 4H, J = 8.1 Hz, Ar-tol), 7.49 (d, 4H, J = 8.1 Hz, Ar-tol), 7.64 (brs 6H, NH3+). IR (ATR) νmax/cm−1 3487, 3059, 1600, 1536, 1495, 1160, 1120, 1032, 1008, 816, 682, 564.
D-alanine p-toluenesulfonate (9)
White solid (quant. yield) 1H-NMR (DMSO-d6): δ (ppm) 1.36 (d, 3H, J = 7.5 Hz, CH3), 2.27 (s, 3H, CH3-tol), 2.47 - 2.49 (m, 1H, CH), 7.10 (d, 2H, J = 8.7 Hz, Ar-tol), 7.46 (d, 2H, J = 8.7 Hz, Ar-tol), 8.14 (brs 3H, NH3+). IR (ATR) νmax/cm−1 3065, 2946, 1747, 1599, 1518, 1495, 1462, 1199, 1157, 1122, 1108, 1036, 1008, 922, 852, 813, 682, 556.
Glycine p-toluenesulfonate (10)
White solid (quant. yield) 1H-NMR (DMSO-d6): δ (ppm) 2.29 (s, 3H, CH3-tol), 3.69 (q, 2H, J = 5.4 Hz, CH2), 7.12 (d, 2H, J = 7.9 Hz, Ar-tol), 7.48 (d, 2H, J = 7.9 Hz, Ar-tol), 8.07 (brs 3H, NH3+). IR (ATR) νmax/cm−1 3051, 2952, 2549, 1747, 1591, 1494, 1438, 1226, 1153, 1117, 1033, 1009, 925, 858, 817, 680, 581, 552, 497.
Acknowledgements
The financial support of this work by the Croatian Science Foundation (grant No. 9310) is acknowledged.