Evaluation of Total Seed Protein Content in Eleven Arkansas Cowpea (Vigna unguiculata (L.) Walp.) Lines ()
1. Introduction
Cowpea [Vigna unguiculata (L.) Walp.] serves multiple purposes for human life. It can be consumed as dry seeds and a vegetable, or used as a cover crop [1] [2] . Industries process cowpeas by both canning and freezing [3] . Cowpea seed grain is used for human consumption as an affordable source of protein and constitutes a supplement fodder to cereal for livestock [4] [5] [6] . In addition, a study carried out by Kushwaha and Kumar [7] stated that cowpea flour can be used to develop high protein biscuits, which would help enhance the nutritional quality of food. The good functional properties of cowpea protein provide plant protein source for people who are suffering allergies to soybean protein.
Cowpea probably helps address the issue of food security, which is a great concern. Food insecurity is accelerated by the rapidly growing world population and the visible effects of climate change, which hamper the livelihood of farmers worldwide [8] . It has been shown that cowpea consumption could have a positive impact on human health [9] . Sreerama et al. [10] reported that cowpea could be used as an ingredient to develop healthy food. Moreover, an increased consumption in cowpea has decreased severe malnutrition up to 100% in children [11] .
The assessment of protein content in cowpea is of interest in order to identify genotypes with high protein content. Itatat et al. [9] assessed eleven cowpea genotypes for seed protein content. Their results revealed that the seed protein content of those cowpea lines ranged from 20.57% to 24.95%. Research performed by Afiukwa et al. [12] on 110 cowpea genotypes exhibited a greater variability than what Itatat reported [9] . Afiukwa et al. [12] have found that the total seed protein content for their genotypes varied from 15.06% to 38.5%, with a mean of 25.99% ± 4.82% in dry seeds. Oke et al. [13] analyzed the protein content in five varieties of cowpea and found that the protein content ranged from 25.80% to 28.95%. Moreover, protein fractions viz. albumins, globulins, prolamins and glutelins of cowpea genotypes showed significant differences according to a study by Gupta et al. [14] . Their analysis on molecular weights of protein bands from 11 cowpea genotypes using SDS-polyacrylamide gel electrophoresis displayed a variation between 10 to 141.3, 15.85 to 147.9, 10 to 125.9, 7.94 to 56.23 and 10 to 79.43 kDa for total proteins, albumins, globulins, prolamins, and glutelins, respectively.
However, the total seed protein contents in cowpea significantly differ among cowpea varieties [9] [12] . Fernandes et al. [15] reported that seed protein content was controlled by three to seven genes with very high narrow-sense heritability (h2) 87.6% in the P1, P2, F1, F2, and backcross populations derived from the cross IT97K-1042-3 × Canap and with a moderate h2 (47.7%) in another cross IT97K-1042-3 × BRS Tapaihum. In addition to the genetic background, location can engender such variability in seed protein content in cowpea [15] . This variability will impact food quality involving cowpea in a way that the physical properties of food such as firmness, springiness, cohesiveness and chewiness of gluten-free rice muffins, for instance, depend on seed protein content [16] . Because cowpea seed protein content has high heritability with few genes, it provides the possibility of developing new high protein cowpea cultivars. Seed protein content is an important parameter in cowpea. Gathering data on this nutritional parameter is crucial; because doing so will help plant breeders choose cowpea breeding lines with high protein contents. The objective of this study is to assess the seed protein content in eleven cowpea genotypes and their expression in differential environment.
2. Materials and Methods
2.1. Plant Materials, Field Experiment, and Seed Sample Preparation
Eleven cowpea genotypes with different seed colors developed by the University of Arkansas were used in this study for evaluation of total seed protein content. Included were 01―1781 (seed color: cream), 07―303 (red), 09―204 (brown eye), 09―208 (pink eye), 09―393 (pink eye), 09―655 (pink eye), 09―714 (pink eye), 09―741 (redholstein), “AR Blackeye #1” (black eye), Early Scarlet (pink eye), and “Ebony” (black) (Table 1).
The field experiment was conducted in three different locations within Arkansas State (Fayetteville, Alma, and Hope) in 2015. A randomized complete block design (RCBD) with two blocks was used for the experiment in each location.
In each plot, cowpea genotypes were planted in four rows 15 feet long, with three feet between rows. Plant spacing within row was four inches. During the growing season, no pesticides or herbicides were sprayed to control pests, diseases, and weeds. The irrigation was rain fed.
The cowpeas were harvested when 90% of pods were dried. The seeds of each cowpea genotype were bulk harvested. The pods were harvested and kept in clean and previously labeled paper bags. A total of 66 samples were collected from the 11 cowpea
![]()
Table 1. Eleven cowpea lines and their multiple comparisons.
*LSMean signifies the Least Square Mean for each of the 11 cowpea genotypes, estimated from JMP Genomics. #Significant test of seed protein content of the 11 cowpea genotypes across three locations two replicates (blocks). The capital letters represent the statistical significance at P = 0.05 level.
genotypes in three locations, with two blocks from each location. The pods of the 66 samples were dried and cleaned. Before measuring seed protein content, each cowpea genotype was further selected for seeds with uniform size and without any insect damage. In order to have a sufficient quantity of seed for the protein analysis, approximately 100 g of seed from each sample were prepared.
2.2. Seed Protein Content Evaluation
Cowpea seed content was measured by analyzing the percentage of Nitrogen by combustion using an Elementar Rapid N III instrument. The estimated 20 g of cowpea seeds were ground and the flour sifted using a sieve of 850 µm, and each 0.1 g of sample was collected and measured for protein content.
At high temperature and in presence of pure oxygen, nitrogen is liberated by combustion. The nitrogen is then isolated from other combustion products. A thermal conductivity detector measures the nitrogen content in the sample [17] . The percentage of nitrogen in each sample was provided, and the total protein content for each sample was estimated by times 6.25% nitrogen [18] .
2.3. Data Analysis
Analysis of cowpea seed protein data was performed by analysis variance (ANOVA) using the general linear models (GLM) procedure of JMP Genomics 7 (SAS Institute, Cary, NC). For comparisons among genotypes, the student T-test was used to perform multiple comparisons for least square mean (LSM) protein content at P = 0.05. The mean, range, standard deviation (SD), standard error (SE) and coefficient of variation (CV) were estimated for seed protein content using “Tabulate”; and the distributions of protein content was also performed using “Distribution” in JMP Genomics 7.
Let Yijk = value of the total seed protein content in the ith location and the jth block for the kth cowpea genotype, for i = 1, 2, 3; j = 1, 2 and k = 1, 2, ・・・, 11. Because there were no replicates in each block, the block was treated as replicates in model for analysis.
The statistical model for the analysis will be the following:
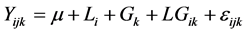
where µ: constant (overall mean), Li: Effect of the ith location (fixed effect) on the mean protein content, Gk: Effect of the kth genotype (fixed effect) on the mean response, LGik: potential joint effect of the ith location and the kth genotype on the mean response, and εijk: experimental associated with the ijkth observation.
The broad-sense heritability (H2) was estimated using the formula.
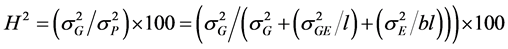 [19]
[19]
where : Genotypic variance;
: Genotypic variance;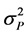 : Phenotypic variance;
: Phenotypic variance; : Genotype × Location variance;
: Genotype × Location variance;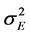 : Variance associated with the experimental error; b: number of blocks within each location;
: Variance associated with the experimental error; b: number of blocks within each location;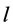 : number of locations.
: number of locations.
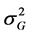 ,
, 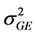 , and
, and 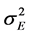 were obtained using the following formulas:
were obtained using the following formulas:
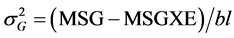 ,
, 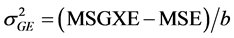 ,
,  where MSG: Mean Square Genotype, MSGXE: Mean Square Genotype × Location, and MSE: Mean Square Error.
where MSG: Mean Square Genotype, MSGXE: Mean Square Genotype × Location, and MSE: Mean Square Error.
The estimates of MSG, MSGXE, and MSE were derived from the ANOVA table.
3. Results and Discussion
In this study, protein content is estimated by using the percentage of total nitrogen after combustion. However, there is evidence that not all nitrogenous compounds are protein so that the estimated protein would not correspond to the true protein content [20] . Despite this limitation, the evaluation of crude protein in legume seeds has proven to be effective in germplasm evaluation, and breeding and genetics studies pertaining to protein content. In soybean, thanks to such a methodology, Warrington et al. [21] reported 4 QTLs associated with crude protein. Similarly, Jadhav et al. [22] identified 4 QTLS associated with crude protein in chickpea using NIR Spectra Alyzer® after calibrating the system with 30 genotypes using the combustion method.
The seed protein content averaged 25.4%, with a range from 23.7% to 27.4%, and had a standard deviation (Std) of 1.94% with 0.24% Std Error, indicating the seed protein content had large variation in the 11 cowpea genotypes (Figure 1).
Significant differences in protein content were observed among the 11 cowpea genotypes (Table 1). Early Scarlet had the highest seed protein content, with 27.4% dried seed weight; 09-204 was second highest (26.9%); and the two were not significantly different each other, but they had total protein content significantly higher than the other genotypes. 01-1781 was third highest in protein content with 25.9% dry seed weight and it was not significantly different from 09-393 (25.9%), 09-208 (25.5%), and 07-303 (25.2%). AR Blackeye #1, 09-714, and Ebony had the same total protein content, with 24.9% dry seed weight, which is significantly different from others but not from 09-655 (24.0%).09-741 had the lowest seed protein content with 23.7% dried seed weight, but was not significantly different from 09-655 (24.0%) (Table 1).
Location effect was detected among the three locations (Table 2 and Table 3).
![]()
Figure 1. Distribution of the total seed protein content among eleven cowpea lines. x-axis presents the seed protein content percentage and the y-axis represents the number of observations.
![]()
Table 2. ANOVA for the total seed protein content among the eleven cowpea genotypes.
![]()
Table 3. Multiple comparisons of the location effect.
*LSMean signifies the Least Square Mean for each of the 11 cowpea genotypes, estimated from JMP Genomics. #Significant test of seed protein content of the 11 cowpea genotypes across three locations two replicates (blocks). The capital letters represent the statistical significance at P = 0.05 level.
Experiment location in Alma exhibited the highest seed protein content; Experiment location in Hope second; and in contrast, experiment location in Fayetteville the lowest, indicating the environment (Location) affected seed protein content in cowpea.
Significant differences were detected among the cowpea genotypes, location, and the interaction of genotype × location (Table 2), suggesting that significant genotype effects existed and genotype by environment effect existed.
From Table 2, MSE (Least Mean Square of Error) was 0.73; MSGXE (Least Mean Square Genotype by Location) was estimated 3.04; and the estimate of MSG (Least Mean Square Genotype) was 7.21. The estimate of the broad sense heritability H2 for cowpea seed protein was 57.8% based on the eleven cowpea genotypes. This relatively low estimate of H2 indicated that factors such as the location (environment) significantly affected seed protein in cowpea.
The genotypes Early Scarlet (27.4%) and 09-204 (26.9%) had the highest protein content among the eleven cowpea breeding lines involved in this study. A significant difference was found in terms of total seed protein content between those lines (P-value < 0.0001). A study performed by Itatat et al. [9] revealed that the genotype “Ife Bimpe” had the highest seed protein content (24.95%) among the cowpea genotypes in their study. In addition, Boukar et al. [23] evaluated the protein contents in cowpea germplasm from IITA (International Center of Tropical Agriculture) genetic resources unit and found an average of 24.7%. Therefore, Early Scarlet and 09-204 could be good parents for breeding purposes for high protein content in cowpea. With respect to the relatively narrow variability in crude protein content among the 11 cowpea genotypes, future study involving a large number genotypes would provide more consistent data for selecting parents for high protein content in cowpea. In addition, further study is needed in order to unravel the protein fractions existing within the cowpea seeds. In addition to the variability in protein content among cowpea cultivars, [14] showed that the prevalence of different protein fractions, mainly consisting of albumins, globulins, prolamins and glutelins, was variable among cowpea lines.
A significant genotype by location interaction (P-value = 0.0002) was found in this study. According to a study by Oluwatosin [24] , the environment accounts for 71% of the variability in protein in cowpea. The results from the research performed by Bliss et al. [25] also indicated a significant genotype by location interaction effect on protein content in cowpea. In addition, Ddamulira and Santos [26] found that protein content in cowpea was significantly affected by the interaction between genotype and environment. Our date support their conclusion, genotype and environment have their contribution to seed protein content in cowpea.
Broad sense heritability is a commonly used parameter in plant breeding [20] . The estimate of H2 represents the proportion of phenotypic variance which is due to genetic effects [27] . In this study, the estimate of such a parameter was 57.8%, which was medium, indicating that protein content can be inherited and can be selected for in the progeny. Inheritance was also affected by environment, which could be explained by the significant genotype × environment interaction. In addition, Noubissie et al. [28] found that the broad sense heritability for seed protein content in common beans varied from 46% to 78%. A study conducted by Ajeigbe et al. [29] indicated a broad sense heritability in cowpea ranging from 56% to 95%. Those results indicate that the estimate of the broad sense heritability dramatically varies among cowpea genotypes and the 57.8% protein content from the present study was reasonable.
4. Conclusion
Cowpea provides cheap protein for human consumption. Dried seeds can be cooked or transformed into flour for multiple purposes. In this study, significant genotype, location, and genotype × location effects were found. The results indicate that protein content was significantly different among cowpea genotypes, and the environment also had significant effect on the total seed protein content in cowpea. The genotypes, Early Scarlet (27.4%) and 09-204 (26.9%) had the highest seed protein content and can be used for breeding to enhance high protein content in cowpea. Further studies will be carried out to determine the different protein fractions in cowpea, and more lines will be evaluated for protein content.