Received 20 March 2016; accepted 15 May 2016; published 18 May 2016

1. Introduction
Rice (Oryza sativa L.) is among the most important crops all over the world and it is consumed as a staple as well as primary source of energy and protein. Rice blast disease, caused by the fungus Pyricularia oryzae, is among the most significant diseases affecting rice cultivation, since it is prevalent in most rice growing regions and causes serious yield losses [1] . Microorganisms including actinomycetes are responsible for the production of approximately 23,000 bioactive secondary metabolites that in turn produce more than 10,000 of these compounds, making up 45% of all bioactive microbial metabolites that have been identified. Among actinomycetes, approximately 7600 compounds came from Streptomyces species [2] . Streptomyces are commonly used in commercial drug industry to produce antibiotics [3] and also known to be a rare source to produce vital compounds for medicine and agriculture [4] . After undergoing various biological processes, Streptomyces are able to successfully control plant pathogenic fungi including P. oryzae by hydrolyzing their cell walls [5] . Secondary, metabolites can be extracted using different solvents and elucidation of the active metabolites and their structures can be performed by various methods such as gas chromatography-mass spectrometry (GC-MS), liquid chromatography-mass spectrometry (LC-MS) and nuclear magnetic resonance (NMR) [6] . The utilization of LC-MS for screening of different compounds together with GC-MS are commonly used today [7] [8] . The efficiency of separation and identification of compounds from complex biological mixtures is very high for GC-MS [9] . This method is mainly utilized for producing fingerprints of volatile compounds which make the process of analysis repetitive [10] [11] . GC-MS can be utilized for separation of volatile or semi-volatile compounds with lower molecular mass [8] . LC-MS is another method through which liquid chromatography is used together with mass spectrometry [9] . It can identify and analyze a large number of compounds, present in small quantities whose molecular mass varies from 10 to 300,000 with different chemical properties.
2. Materials and Methods
2.1. Preparation of Crude Extract
Streptomyces sp. isolate UPMRS4 (KT693185), identified from preliminary screening, was cultivated on potato dextrose agar (PDA) (Difco™ Company USA) and incubated at 28˚C for seven days. The agar was cut into small pieces (about 1 cm) to be used for extraction with solvents of different polarity (chloroform, ethyl acetate, methanol, acetone, ethanol, diethyl ether and water) and placed into 500 ml top capped bottles with 250 ml of each solvent. The mixtures of agar and each solvent were shaken in a rotating shaker at 150 rpm overnight at 30˚C. This was followed by centrifugation of the mixtures at 10,000 rpm (Avanti J-26 XPI centrifuge, Beckman Coulter, USA) for 10 min to separate from supernatants. A vacuum filtration pump was used to filter the extracts with two layers of Whatman No.1 filter papers (ALBERTR) before drying with a rotary evaporator at 40˚C. The concentrated powder of each extract was regarded as crude sample and stored at −20˚C for further use. Crude extracts were dissolved in 50% methanol for antifungal, bioassay and chromatography analysis.
2.2. Antifungal Bioassay
Agar well diffusion method was used to screen the crude extracts for any antifungal activity following Patel et al. [12] with some modifications. Antifungal bioassay was done on 9 cm Petri plate with 20 ml of PDA. The fungal pathogen used was P. oryzae (KT693184), obtained from the Department of Plant Protection, Universiti Putra Malaysia. A 5 mm fungal plug was cut from the leading edges of a seven-day-old pure culture of P. oryzae, and was put in the center of the plate. A 5 mm diameter well was made at 2.5 cm from the fungal plug and another well at the opposite side, followed by pipetting 30 µl aliquot of each crude extract into the two wells on each plate. Control plates contained wells of filter-sterilized methanol 50% and sterile distilled water. Radial growth was recorded after incubation for 10 days. The radial growth of fungal colony was recorded with a meter ruler along two diagonal lines drawn on the reverse side of each plate. Three plates with two radial measurements per plate were conducted and the experiment was repeated twice. The percent inhibition of radial growth (PIRG) of each treatment compared to control was computed utilizing the formula below:
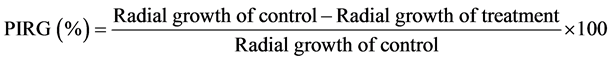
2.3. Determination of Effective Inhibitory Concentration (EIC)
Crude extract which showed the highest antifungal activity was selected to determine the effective inhibitory concentration (EIC) at 50% inhibition of mycelial growth. The EIC was determined using the antifungal bioassay described in section 2.2 above with concentrations of 1.56, 3.125, 6.25, 12.5, 50 and 100 µg/ml.
2.4. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
Crude extract which showed the highest antifungal activity was subjected to aShimadzu GC-17A attached to a Shimadzu GC-MS-QP5050A system. The column used was a Phenomenex Zebron ZBFFAP ultra-low-bleed Bonded Polyethylene Glycol fused capillary column of 30 ml × 0.25 mm I.D × 0.25 µm film thickness. Split ratio 20 injection was performed. Helium was the transporter carrier gas with a stream flow rate of 0.7 ml/ min. The column temperature was kept at 70˚C for 3 min, then modified at 10˚C/min to 90˚C via programming and finally modified at 5˚C/min to 230˚C. The inlet and detector temperatures were 230˚C and 250˚C, respectively, while the dissolvable deferral (solvent delay) was 5.75 min [13] .
2.5. Liquid Chromatography-Mass Spectrometry (LC-MS/MS) Analysis
An AB Sciex 5500QTrap (Linear Quadrupole Hybrid Ion Trap Mass Spectrometer, AB Sciex, Toronto, Canada) mass spectrometer operating in electrospray ionization (ESI) negative mode and hyphenated with an Agilent 1290 ultra-high performance liquid chromatography system was used. The high purity nitrogen gas for the mass spectrometer was set at 40 psi for source gas, 40 psi for the heating gas and HIGH for collision gas with a source temperature of 500˚C. The setting for electrospray ionization voltage was set to 4500 kV. The collision energy to attain fragmentation was set at 35 eV with a spread of ±15 eV. Mass range for MS/MS scan was set from 50 - 1000 m/z while mass range for full scan was set from 100 - 1000 m/z while scan speed was set at 1000 m/z per second.A Phenomenex Synergi Fusion RP (100 mm × 2.1 mm i.d., 3 um particle size, Phenomenex, CA, USA) was used to obtain separation. The mobile phase was made up of aqueous ammonium formate (5 mmol/L) with 0.1% formic acid (solvent A) and acetonitrile with ammonium formate (5 mmol/L) with 0.1% formic acid (solvent B). The compounds were separated with the following linear-programmed solvent gradient: 0 min (10% B), 10 min (95% B), 2 min (95% B) then equilibrating back to 10% B for 3 min. The flow rate for the column was set at 0.25 mL/min while the column temperature was set at 40˚C and injection volume at 10 uL.
2.6. Statistical Analysis
Statistical analysis was done using one way Analysis of Variance (ANOVA) at confidence level 95% using SAS, 2003. Comparison of means was conducted with Duncan.
3. Results
3.1. Antifungal Bioassay
The inhibition rates of different solvent extracts of Streptomyces sp. isolate UPMRS4 on the mycelia growth of P. oryzae are shown in Figure 1. All extracts showed some effectiveness on the tested pathogen ranging from 55.3% to 98.33%. Ethyl acetate extract showed the highest inhibition significantly different from other solvent extracts. Based on this result, ethyl acetate was chosen for the rest of the experiments. Figure 2 shows the 98.33% inhibition of mycelial growth of P. oryzae compared to the control.
3.2. Determination of Effective Inhibitory Concentration (EIC)
All the tested concentrations of ethyl acetate crude extract exhibited inhibition on mycelial growth of P. oryzae. The suppressive effect increased with the increase in concentration at 1.56 to 100 μg/ml (Figure 3). Concentration of 100 µg/ml showed significantly higher inhibition of the pathogen (98.33%) followed by 50 µg/ml (78.33%) and 25 µg/ml (75.66%). There was no significant difference between the three of them but there was significant difference compared to that of 12.5 µg/lm (64%). Lower concentrations of 6.25, 3.125 and 1.562 µg/ml were also able to inhibit mycelia growth at 56.66%, 56.16% and 55.3%, respectively.
![]()
Figure 1. Effect of Streptomyces sp. crude solvent extracts against P. oryzae using well diffusion method.
![]()
Figure 2. Effect of ethyl acetate crude extract on P. oryzae; (a) control and (b) treated.
![]()
Figure 3. Effect of Streptomyces sp. ethyl acetate extract on P. oryzae.
3.3. Analysis of Volatile Compounds from Ethyl Acetate Crude Extract Using Gas Chromatography-Mass Spectrometry (GC-MS)
The result related to GC-MS dissection showed the presence of 22 different volatile compounds in the ethyl acetate crude extract (Figure 4). The major compounds present in the extract as indicated by some highest peaks were identified as 1) Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro-3-(2-methylpropyl) with the retention time 16.748; 2) Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro-3-(phenylmethyl) with the retention time 22.148; 3) ergotamine with the retention time 21.783.
3.4. Liquid Chromatography-Mass Spectrophotometry (LC-MS/MS) Analysis
The presence of peaks that appeared in the chromatogram showed the presence of 35 different compounds from ethyl acetate crude extract (Figure 5). Four of the compounds in the extract indicated by highest peaks were identified as 1) Amicomacin(C20H29N3O7), with peak at retention time of 3.25 and molecule weight 421.68 (Figure 6); 2) Fungichromin (C35H58O12), with peak at retention time of 11.85 and molecule weight 670.49 (Figure 7); 3) N-Acetyl-D, L-phenylalanine (C11H13NO3), with peak at retention time of 2.98 and molecule weight 206.38 (Figure 8) and (4) Rapamycin, (C51H79NO13), with peak at retention time of 6.61 and molecule weight 912.85 (Figure 9).
![]()
Figure 4. Gas chromatography-mass spectrophotometer (GC-MS) analysis of ethyl acetate crude extract.
![]()
Figure 5. Liquid chromatography-mass spectrophotometry (LC-MS/MS) analysis of ethyl acetate crude extract.
![]()
Figure 6. Detection of amicomacin by LC-MS/MS from ethyl acetate extract.
![]()
Figure 7. Detection of fungichromin by LC-MS/MS from ethyl acetate extract.
4. Discussion
The use of antagonistic microorganisms like Streptomyces spp. is an appropriate approach to effectively control
![]()
Figure 8. Detection of N-acetyl-D, L-phenylalanine by LC-MS/MS from ethyl acetate extract.
![]()
Figure 9. Detection of rapamycin by LC-MS/MS from ethyl acetate extract.
diseases in plants [14] [15] . In recent times, Sabaratnam and Traquair [16] have indicated that Streptomyces sp. isolate Di-944 shows the same effectiveness as the fungicide, oxine benzoate, when it is used as drenching to control Rhizoctonia damping-off of tomato. Screening for the active crude extract using different solvents is crucial to find out the best solvent which extracts the highest amounts of bioactive compounds. This is supported by Khamna et al. [17] and Kobayashi et al. [18] studies which reveal that ethyl acetate extract display a greatest antimicrobial activity against bacteria and fungi.
In the present study, the result demonstrates the presence of 22 different volatile compounds in the ethyl acetate crude extract. Recent studies have illustrated that a number of these compounds can lead to direct inhibition of fungal and bacterial pathogens. Pyrrolo 1,2-a pyrazine group is a group of potent naturally occurring antibiotics from various Streptomyces species, which are among the most promising types of lead compounds [19] . Melo et al. [20] also isolate such compounds [Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro-3-(2-methyl-propyl) and Pyrrolo[1,2-a] pyrazine-1,4-dione, hexahydro-3-(phenylmethyl)] from the fungus Mortierella alpina and report their antibacterial activity against Escherichia coli, Pseudomonas aeruginosa and Enterococcus faecalis. These two compounds have been isolated from endophytes, Epichloe spp. and Neotyphodium spp. of grasses in cold regions which have the capability to protect plants from worms and phytopathogens [21] [22] .
Based on the chemical constituents identified using LC-MS/MS, Azumi [23] reports that amicoumacin shows plant growth inhibitory activity against barnyard millet. The most significant finding that emerged from the analysis of his data is that fungichromin (pentamycin). Earlier research has mentioned that fungichromin is found in many species of microorganisms such as S. cellulose, S. fradiae, S. griseus, S. roseoluteus, and Verticilliumcin namomeum sub sp. cinnamomeum [24] .
Our findings revealed that rapamycin (sirolimus), it was initially found to be an antimicrobial compound to bust activity against Candida albicans [25] . Following that discovery, rapamycin was shown to have strong antifungal activity against several human fungal pathogens including Cryptococcus neoformans, Aspergillus fumigatus, Fusarium oxysporum, and many pathogenic Penicillium species [26] , and it was eventually found to have strong immunosuppressive activity [27] . Bastidas et al. [28] indicated that rapamycin inhibited up to 80% of growth at concentrations above 6.26 μg/ml, 12.5 μg/ml, and 100 μg/ml for Phycomyces blakesleeanus, Rhizopus oryzae, and Mucor circinelloides, respectively.
In conclusion, the findings of the current study showed that Streptomyces sp. isolate UPMRS4 had significant antifungal activity against rice blast pathogen, P. oryzae. Ethyl acetate crude extract showed the highest inhibition on the mycelial growth of P. oryzae and the EIC was determined as 1.562 μg/ml. GC-MS analysis showed that ethyl acetate crude extract of isolate UPMRS4 contained macrolides compounds, Pyrrolo[1,2-a] pyrazine-1,4-dione group while LC-MS/MS demonstrated the presence of fungicidal compounds such as amicoumacin, fungichromin, N-acetyl-D, L-phenylalanine and rapamycin. These results indicate that Streptomyces sp. isolate UPMRS4 had the potential to be developed as a biocontrol agent to control rice blast pathogen, P. oryzae.
NOTES
![]()
*Corresponding author.