1. Introduction
Root-knot nematodes were included within the genus Meloidogyne and belonged to a relatively small but important polyphagous group of highly adapted obligate plant pathogens. They were among the most hazardous soilborne plant parasites and were responsible for large economic losses in a wide variety of crops worldwide. Typically, they reproduced and fed within plant roots and caused small to large galls or root-knots. Meloidogyne incognita was currently causing problems on many economic crops in conventional as well as organic farming. For several decades, the management of plant-parasitic nematodes has been dominated by the use of synthetic nematicides. Their application is problematic because of negative environmental impacts and, consequently, many nematicides have been withdrawn from the market, so new control strategies are needed. Understanding the mechanisms and factors involved in host location could provide powerful opportunities for controlling the nematode by disrupting its host finding behaviour.
Steiner [1] proposed that plant-parasitic nematodes located their hosts by chemoreception. Subsequently, a series of experiments were conduced on the chemotaxis of nematodes in response to host roots [2] -[5] , pH [6] , carbon dioxide [7] [8] , temperature [9] [10] , sex pheromones [11] and inorganic ions [12] -[14] . The chemoreception of nematodes in response to different attractants has been reviewed by Perry [15] [16] . As salts exist naturally in the soil and are easily altered by crop fertilisers, understanding the effects of salts on nematode orientation will be important as a potential basis for developing new management approaches.
Salt ions of Na+, Mg+, Cl− and OAc− have been reported to attract Rotylenchulus reniformis [17] , whilst K+, 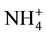 , Cs+,
, Cs+, 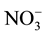 and Cl− are strongly repellent to infective second-stage juveniles (J2) of M. incognita [13] . Saux and Quénéhervé [14] noted that calcium salts had no effect on the juvenile orientation of M. incognita, whilst ammonium salts and ammonium nitrate were strongly repellent. By contrast, the orientation of R. reniformis depended on the constitutive anion of the salts, e.g., chloride salts were found to be repellent but sulphate and nitrate salts were attractive. Ditylenchus destructor had attraction or repellent for some ions, such as Cl−,
and Cl− are strongly repellent to infective second-stage juveniles (J2) of M. incognita [13] . Saux and Quénéhervé [14] noted that calcium salts had no effect on the juvenile orientation of M. incognita, whilst ammonium salts and ammonium nitrate were strongly repellent. By contrast, the orientation of R. reniformis depended on the constitutive anion of the salts, e.g., chloride salts were found to be repellent but sulphate and nitrate salts were attractive. Ditylenchus destructor had attraction or repellent for some ions, such as Cl−, 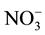 ,
, 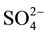 ,
, 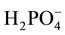 and
and 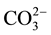 [18] . The present study aimed to investigate the chemotaxis of M. incognita in response to 48 different salts.
[18] . The present study aimed to investigate the chemotaxis of M. incognita in response to 48 different salts.
2. Materials and Methods
2.1. Nematodes
The diseased roots of tomatos cv. Digny powder were collected from a field in Wuwei, Gansu, China infested with M. incognita [19] . Fresh egg masses were picked with needle from tomato roots under anatomical lens and put into Petri dish. Subsequently, egg masses were sterilized with 0.5% NaOCl2 for 3 min, washed three times with distilled water, and placed into 24 holes plate for four days hatching at 25˚C. Then the 2nd stage juveniles (J2) were used bioassay.
2.2. Salts
The salts tested were NH4Cl, NaCl, KCl, CuCl2∙2H2O, FeCl3∙3H2O, CsCl, (NH4)2SO4, Na2SO4, K2SO4, MnSO4∙H2O, FeSO4∙7H2O, CuSO4∙5H2O, (NH4)2SO4∙FeSO4∙6H2O, NH4NO3, NaNO3, KNO3, Ba(NO3)2, Mn(NO3)2, Na2HPO4, NaH2PO4, (NH4)2HPO4, NH4H2PO4, KH2PO4, K2HPO4, NH4HCO3, (NH4)2CO3, Na2CO3, K2CO3, KHCO3, CaCO3, NaHCO3, CH3COOH, CH3COONa, CH3COOK, CH3COONH4, NH4SCN, KSCN, NaSCN, NaOH, KOH, Na2Wo4∙2H2O, C3H6O3, C4H6O6, C6H8O7, C6H5Na3O7, C7H5NaO3, C10H14N2Na2O8 and CO(NH2)2. For each salt, six concentrations with pre experiment were tested: 2 × 10−2, 1 × 10−2, 0.5 × 10−2, 0.25 × 10−2, 0.125 × 10−2 and 0.0625 × 10−2 mol∙L−1.
2.3. Bioassay
The experimental set up used in this study was modified from Wuyts [20] . In brief, 5-cm-diam. Petri dishes were divided into 16 sections in two circles, viz. an inner and outer circle (Figure 1). The Petri dishes were filled with 0.8% agarose. In the outer circle of each dish, 50 µl of the salt being tested and distilled water were inoculated on opposite sides (S and W, respectively, in Figure 1) and incubated for 1 h at 25˚C. Mixed stages of 30 M. incognita J2 in 5 µl were eventually transferred to the centre of the test arena and incubated at 25˚C darkness condition for 5 h. After this period, movement of nematodes was stopped by spraying the plates with ethanol. The numbers of nematodes from section 1 - 8 were counted. Five plates were tested for each concentration and the
![]()
Figure 1. Test arena for investigating the chemotaxis of Meloidogyne incognita. S: salts; W: distilled water; N: nematodes.
control in which the salt was replaced by the distilled water. All the experiments were repeated four times.
2.4. Data Analysis
The chemotactic index was defined as a positive number (attractant) or negative (repellent) number ranging from +2 cm to −2 cm (which means the average distance of the nematodes travelled from the centre of Petri dish). The chemotactic index was calculated based the equation as followed:
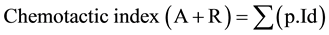 [14]
[14]
where: p = nematode proportion in the selected sections 1 - 8 (the number of nematodes in these sections expressed as a percentage of the total number of nematodes inoculated onto the Petri dish); Id = distance index (2 cm for section 1 - 2, 1 cm for section 3 - 4, −1 cm for section 5 - 6, −2 cm for section 7 - 8).
The Duncan’s new multiple range test was used to analysis the significance between treatment and control (P < 0.05).
3. Results
3.1. The Chemotactic Responses of M. incognita to Different Salts
The chemotactic responses of M. incognita to 48 different salts at six concentrations are shown in Figures 2-6. In general, the salts containing Cl− and SCN− anions were repellent to M. incognita (Figure 2 and Figure 3). Other salts that comprised the same anions had different chemotactic responses, among which M. incognita was repellented to ammonium salts that included Ba(NO3)2, NH4NO3, Mn(NO3)2 (Figure 4), and hydrogen-phos- phate salts that included KH2PO4, K2HPO4, and bicarbonate salts that included Na2CO3, K2CO3, (NH4)2CO3, KHCO3 (Figure 5), and hydroxyl salts that included KOH, NaOH, and organic acid that included CH3COOH, C3H6O3 and C4H6O6 (Figure 6).
The salts comprising the same cation had different chemotactic responses, some showed attraction, others repellent. The effects of different salts with the same cation were not consistent. The order of repellence for the anion was SCN− > 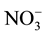 > Cl− > OH− >
> Cl− > OH− > 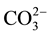 >
> 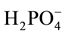 > organic acid >
> organic acid >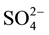 . Nematodes were more strongly repellent to salts containing SCN− and
. Nematodes were more strongly repellent to salts containing SCN− and ![]() than to those with other anions.
than to those with other anions.
3.2. The Chemotactic Indexes of M. incognita in Response to Different Salts
For each salt the chemotactic indexes varied with the concentration tested. The highest chemotactic index was 0.53 (the maximum value would be 2.0) and the lowest chemotactic index was −1.09 (minimum −2.0) from all the salts tested. Nematodes were significantly repellent to salts containing Cl− and SCN− anions at all test concentrations (Table 1). The chemotactic index of NH4Cl, KCl, CsCl, (NH4)2SO4, Na2SO4, FeSO4∙7H2O, Ba(NO3)2, NH4H2PO4, K2CO3, KHCO3, NaOH and C7H5NaO3 at six concentrations were found no significant difference. CuCl2∙2H2O and CH3COONH4 only the lowest concentration 0.0625 × 10−2 mol∙L−1 gave attraction responses, while other concentration had repellence.
If comparing the repellent or attraction responses of M. incognita to the same salts at different concentration,
![]()
Figure 2. Effect of salts containing chlorine anion on the chemotaxis of M. incognita.
![]()
Figure 3. Effect of salts containing thiocyanate anion on the chemotaxis of M. incognita.
![]()
Figure 4. Effect of salts containing nitrate anion on the chemotaxis of M. incognita.
![]()
Figure 5. Effect of salts containing bicarbonate or carbonate on the chemotaxis of M. incognita.
the chemotactic index of KCl, Ba(NO3)2, NH4NO3, Mn(NO3)2, (NH4)2CO3, CH3COOH, and C4H6O6 increased with increasing concentration, whilst for the other salts tested the concentration did not influence nematode chemotaxis significantly.
![]()
Figure 6. Effect of organic acid on the chemotaxis of M. incognita.
4. Discussion
Application of fertiliser is a standard practice in production [21] [22] and, as inorganic or organic salts in the soil are easily altered by crop fertilisers, understanding the effects of inorganic or organic salts on nematode orientation will be important as a potential basis for disrupting nematode orientation. The paper studied the effect of 37 inorganic salts on M. incognita movement and first reported 11 organic salts on M. incognita chemotactic responses, such as CH3COOH, CH3COONa, CH3COOK, CH3COONH4, CH3COOH, CH3COONa, CH3COOK, CH3COONH4, C3H6O3, C4H6O6, C6H8O7, C6H5Na3O7, C7H5NaO3, C10H14N2Na2O8 and CO(NH2)2.
Previous studies showed different nematodes had different attraction or repellent for some cations and anions. Saux and Quénéhervé [14] showed that Na+, Mg2+, Cl− and OAc− had attraction to Rotylenchulus reniformis, and the chemotactic responses was governed more by the constitutive cation than by the constitutive anion. Ditylenchus destructor was attracted to salts that included Cl−, and![]() , whereas salts with
, whereas salts with ![]() and
and ![]() anions were repellent, but the salts comprising
anions were repellent, but the salts comprising ![]() had almost no effect on the nematode movement [18] . Castro showed that K+,
had almost no effect on the nematode movement [18] . Castro showed that K+, ![]() , Cs+,
, Cs+, ![]() and Cl− had strong repellent to J2 of M. incognita [13] . The current study provides information on the response of M. incognita to a range of inorganic salts. It demonstrates the repellent effect of salts containing Cl− and SCN−. The effects of cations on movement of M. incognita are not consistent, the same cations in different salts eliciting different chemotatic responses. We assume that the chemotaxis of different species of nematodes may show different responses to either anions or cations. Both CO (NH2)2 and salts comprising
and Cl− had strong repellent to J2 of M. incognita [13] . The current study provides information on the response of M. incognita to a range of inorganic salts. It demonstrates the repellent effect of salts containing Cl− and SCN−. The effects of cations on movement of M. incognita are not consistent, the same cations in different salts eliciting different chemotatic responses. We assume that the chemotaxis of different species of nematodes may show different responses to either anions or cations. Both CO (NH2)2 and salts comprising![]() , which are components of the most frequently used fertilisers, have a very weak effect on the movement of M. incognita. Obviously, salts containing Cl− and SCN− are repellents for M. incognita. When combined with a chemical nematicide these salts may increase the effect of the nematicide and therefore reduce the dosage required for an efficient control of the nematode.
, which are components of the most frequently used fertilisers, have a very weak effect on the movement of M. incognita. Obviously, salts containing Cl− and SCN− are repellents for M. incognita. When combined with a chemical nematicide these salts may increase the effect of the nematicide and therefore reduce the dosage required for an efficient control of the nematode.
Castro showed the repellent effect of some cations and anions to infective second-stage juveniles of M. incognita, with the order of repellence as K+ > Cs+, ![]() and
and ![]() > Cl− [13] . The current study demonstrated that SCN− had the strongest chemotactic responses. The order of repellence for the anion was SCN− >
> Cl− [13] . The current study demonstrated that SCN− had the strongest chemotactic responses. The order of repellence for the anion was SCN− > ![]() > Cl− > OH− >
> Cl− > OH− > ![]() >
> ![]() > organic acid >
> organic acid >![]() . Saux and Quénéhervé [14] reported Ca2+ almost had no effect on J2 of M. incognita, while NH4NO3 showed the strong repellence. This paper demonstrated that the salts comprising the same cation had different chemotactic responses. Some salts showed attraction, others repellent, among which M. incognita was repellented to ammonium salts that included Ba(NO3)2, NH4NO3, Mn(NO3)2, and hydrogen-phosphate salts that included KH2PO4, K2HPO4, and bicarbonate salts that included Na2CO3, K2CO3, (NH4)2CO3, KHCO3, and hydroxyl salts that included KOH, NaOH. First report had repellent response organic acid for M. incognita was C2H4O2, C3H6O3 and C4H6O6.
. Saux and Quénéhervé [14] reported Ca2+ almost had no effect on J2 of M. incognita, while NH4NO3 showed the strong repellence. This paper demonstrated that the salts comprising the same cation had different chemotactic responses. Some salts showed attraction, others repellent, among which M. incognita was repellented to ammonium salts that included Ba(NO3)2, NH4NO3, Mn(NO3)2, and hydrogen-phosphate salts that included KH2PO4, K2HPO4, and bicarbonate salts that included Na2CO3, K2CO3, (NH4)2CO3, KHCO3, and hydroxyl salts that included KOH, NaOH. First report had repellent response organic acid for M. incognita was C2H4O2, C3H6O3 and C4H6O6.
Salts not only affect nematode movement but also can affect nematode survival. The nematicidal activity of ammonia has been known for a long time. Among 10 ammonia-releasing compounds tested, NH4OH, (NH4)2HPO4 and NH4HCO3 showed marked nematicidal activities in pot experiments [23] . Ammonium sulphate applied with alkaline stabilized biosolid (ASB) significantly reduced the root-galling index of tomato plants infested with M. javanica compared with that of plants grown in soil treated with ammonium sulphate or ASB alone [24] . Although ![]() is not nematicidal, it can form NH3, which is toxic to nematodes in alkaline soil. It is possible that the salts containing cation
is not nematicidal, it can form NH3, which is toxic to nematodes in alkaline soil. It is possible that the salts containing cation ![]() and an anion that elicits a repellence response, e.g. Cl− or SCN−, may have a better control effect than salts comprising other ions. Further studies are needed on this aspect.
and an anion that elicits a repellence response, e.g. Cl− or SCN−, may have a better control effect than salts comprising other ions. Further studies are needed on this aspect.
![]()
![]()
Table 1. Significant tests and its chemotactic index of different salt ions at different concentrations to Meloidogyne incognita.
Note: The data in the figure are mean. Different lowercase letters within the same row show significant differences at P < 0.05 levels by Duncan’s new multiple range test.
5. Conclusion
In the present study, the chemotaxis of M. incognita in response to different concentration of salts is variable. For most salts tested, such as KCl, Ba(NO3)2, NH4NO3, Mn(NO3)2, (NH4)2CO3, CH3COOH and C4H6O6, their chemotactic indices increased with the increasing concentration, whilst for some salts tested their concentration did not influence nematode chemotaxis significantly.
Acknowledgements
This work was supported by the Special Fund for Public Benefit (Agriculture, No. 201103018), and the National Natural Science Foundation of China (No. 31000845).
NOTES
*Corresponding author.