Expected Radial Column Density of Methylcyanopolyynes in CW Leonis (IRC+10216) ()
1. Introduction
New observational evidences in some interstellar mediums (ISM) such as circumstellar shells and cold nebulae, it has been recently reported in Sagittarius B2, HL Tauri, Rho Ophiuchi cloud complex and Orion KL, between others, show the presence and abundance of different molecular species [1] -[8] . Large molecules in heterogeneous astronomical environments is one of the main goals of the frontier Astrochemistry, where project as ALMA (Atacama Large Millimeter Array) is being implemented in the south hemisphere, in order to reach new molecular observations and therefore, new explanations of complex molecular systems such as fullerenes in the ISM originated from long linear molecular compounds such as cyanopolyynes.
Several smaller members of the polyynic series have been detected in the ISM, particularly in cold circumstellar envelopes surrounding carbon-rich stars. These unsaturated carbon chains comprise at one end by hydrogen or methyl group and a CN group in the other extreme, a classical electron acceptor group [9] . The rotational spectra of smaller members of the simplest cyanopolyynes up to HC17 N are well known at the laboratory [10] , but under hard experimental conditions and extreme detection sensitivity. However, HC11N has been the last-discovered large linear interstellar molecule [11] [12] . Whenever spectral measurements of these types of molecular wires involving the CN group [13] [14] from various interstellar regions have been reported since 1978 up to date, the astronomical observations have systematically proved the difficult to detect molecular wires longer than five units.
These linear oligomeric molecular structures are constituted by an electron-donor group (D), in this case CH3 group, a molecular wire bridge (W), represented by the unsaturated carbon chain (-[C≡C]n-), and the CN electron-acceptor group (A) [15] -[17] . By following, when these molecules are formed an electronic charge transfer process occurring from D to A through W, i.e., materialized the dipole moment. In several previous studies [18] - [21] , we have developed an one-dimensional molecular model for these systems based on the electronic conduction properties of linear conjugated oligomeric compounds of the D-W-A type. These studies have allowed us to focus our particular attention on these new interstellar cyanopolyynes.
In the present work, we have extended our cyanopolyynes study [19] to the methylcyanopolyynes, in order to find out the expected radial column density of new species to be present in CW Leonis (IRC +10216). Geometrical and dipole moment parameters calculated from Ab initio molecular orbital calculations, permitted us to determine the molecular resistances, necessary data to correlate the radial column densities in a similar trend previously observed in cyanopolyynes species [19] .
2. The One-Dimensional Conduction Model
Based on our one-dimensional conduction model for D-W-A molecular systems developed some years ago [9] [18] , we have determined the molecular resistance of the first fourteen methylcyanopolyynes and the linear molecular resistivity. Therefore, the dipole moment of every linear nth oligomer (mn) can be represented as [9] :
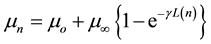 (1)
(1)
where mo is the dipole moment of the first compound of the oligomeric series without a bridge unit (n = 0), m¥ is a molecular constant of the oligomeric series at the limit value for L®¥, L(n) is the molecular wire length of the nth oligomer (-[C≡C]n-), and g is the molecular wire conduction constant. Thus, the inner resistance of the molecular wire can be depicted as follow [9] :
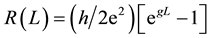 (2)
(2)
where (h/2e2) = 12.91 kW. However, in order to separate the linear and non linear contributions of the molecular wire resistance, we can expand R(L) in Equation (2) as a Maclaurin series:
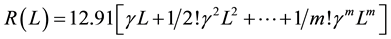 (3)
(3)
where the first term defines the linear contribution given by 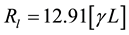 (kW) and the remainder terms of the series define the non linear contribution of the molecular wire resistance. Thus, the linear resistivity (r), an intrinsic property of the conductor wires, can be calculated as r = Rl・(S/L), where S is the molecular wire cross-section estimated to be 4.5 Å2 [18] .
(kW) and the remainder terms of the series define the non linear contribution of the molecular wire resistance. Thus, the linear resistivity (r), an intrinsic property of the conductor wires, can be calculated as r = Rl・(S/L), where S is the molecular wire cross-section estimated to be 4.5 Å2 [18] .
3. Results and Discussion
The best molecular geometry optimization and the electronic charge distribution as a function of the length of the molecular wire for the methylcyanopolyynes under study were obtained by means of the GAUSSIAN Quantum Mechanics Program software [22] . From this calculation program we have used the restricted Hartree-Fock (RHF) approach and the 6-311G* basis set, up to reach the best linear equilibrium geometry. All these oligomeric compounds, from n = 1 to 14, have been well described by means of these molecular-orbital calculations.
However, it is well known fact by experimentalist the inherent difficulties that these cyanopolyynes present for laboratory synthesis according to increase the length of the molecular series, due to high molecular instability, as well as, hard experimental conditions in order to reach dipole moment measurements in vacuum. Therefore, it is not possible to compare systematic dipole moment measurements to our or other calculations, however, the fresh results providing by the recent Astrochemistry research can open new expectative in a near future.
In spite of the scarce studies on this subject, our results permit to project the interstellar chemistry of these particular methylcyanopolyynes [9] [19] . Hence, L(n) as the molecular wire lengths and mn as the dipole moments of these methylcyanopolyynes were calculated using this theoretical approach (Table 1). Figure 1 shows the expected interdependence between mn and L(n) in agreement to the molecular model developed for these molecular wires [9] . In this Figure, the observed trend is better than r2 > 0.999 and the final parameters described by Equation (1) in Figure 1 are presented in Table 2.
Furthermore, from Figure 1 we can observe how this molecular series converge to a maximum dipole moment as function of the polyynic wire in a similar behavior than the cyanopolyynes [19] . Thus, according to Equation (1), the observed limit is determined by (mo + mµ), where the dipole moment in this oligomeric series converge to 8.21 ± 0.01 (Debyes).
Therefore, the oligomeric series under study have been extended to the first fourteen species, where after the CH3C29N compound, the dipole moment does not change significantly.
On the other hand, the g molecular wire conduction constant of methylcyanopolyynes exhibits a similar behavior to the cyanopolyynes [19] . Certainly, the methyl group induces a slight perturbation to the molecular wire nevertheless the observed change is lower than 3%.
Thus, by means of this g conduction constant, we have determined the molecular resistances as a function of the wire length according to Equation (2). Table 3 depicts the molecular resistance of these methylcyanopolyynes and we have determined a linear resistivity of 74.9 mW/cm, similar to cyanopolyynes (72.6 kW/cm) and other molecular wires previously reported [19] .
Based on these results, we have used the molecular resistance as a criterion for chemical reactions feasibility of these linear molecular species [19] . Thus, the molecular resistance to the internal charge transfer emerges as
![]()
Figure 1. Dipole moments of the methylcyanopolyynes (n = 0 to 14) versus the molecular wire lengths in the RHF approach and 6-311G* basis set.
![]()
Table 1. Molecular wire lengths and dipole moments of the Methylcyanopolyynes according to Gaussian calculations in the RHF approach and the 6-311G* basis set.
![]()
Table 2. Dipole moment parameters of the Methylcyanopolyynes.
![]()
Table 3. Inner molecular resistance of the Methylcyanopolyynes.
an indicator of the polarity strength of the ground state during the molecular formation process for every one molecule of the polyynic series. Consequently, every new polyynic unit extension of the molecular wire of the methylcyanopolyynes gradually presents an additional resistance to the internal charge transfer process and, subsequently, their reaction feasibility necessarily decreases, weakening the attraction force of the CN electron-acceptor group trough the molecular wire.
Therefore it is not possible to expect long wire cyanopolyynes when its internal charge transfer activation energy is higher than the thermal energy of the reaction bulk, in other words, when the dipole moment reaches infinitesimal changes between two molecules of the series. This observation is particularly interesting to the interstellar cold dense-clouds. In this case the molecular reactivity for every molecule of the series must be understood how a dependent parameter of the sequential reaction scheme and under the same LTE conditions in a bulk delimited by low-temperature dense-cloud regions.
Therefore, the role of the CN group in the chemical reactions associated to the cyanopolyynes synthesis is determined by the electronic feasibility of the charge transfer between the molecular wire and the electron acceptor group. Thus, we can expect a relationship between the oligomeric species distribution and the molecular resistance to the internal charge transfer of the molecular wires that determines the final probability of the molecular array density under LTE.
IRC +10216 or CW Leonis, a carbon-rich star embedded in a thick dust envelope, has been a specific natural reactor of molecular reactions. This stellar source has been well studied both observationally and theoretically and Millar et al. [7] have developed chemical models in order to reproduce detailed radial distributions of different molecules. In particular, they report the cyanopolyynes chemistry based on the positive ion-molecule and neutral-neutral reactions leading to the production of these oligomers. Reactions including the radical CN and hydrocarbons were involved in the formation of cyanopolyynes, as they are in dense clouds, but reactions between the radical C2H and smaller cyanopolyynes were far more important in the IRC +10216 envelope chemistry.
Radial column densities for the cyanopolyynes in IRC +10216 are well represented by the Millar et al. model [7] , which includes several cyanopolyynes and the only three observed methylcyanopolyynes. Therefore, we have made use of the radial column densities of these last reported oligomers [7] . In Figure 2, we can appreciate the methylcyanopolyynes expected behavior between radial column density and the molecular resistance. A very good linear correlation can be observed in the logarithmic scale in a similar behavior than the cyanopolyynes previously reported [19] . Therefore, this result in a new molecular series as the methylcyanopolyynes gives a new basement to our hypothesis respect to the chemical reaction feasibility in terms of the molecular wire length. Furthermore, the present methodology determines a new approximation to the radial column density estimation for astronomical observations in order to project the presence of new molecular species of these oligomeric varieties.
Thus, based on this linear correlation we have estimated the expected molecular distributions through the radial column density of this series from the relationship established by Figure 2. By following the radial column density (RCD) of these methylcyanopolyynes can be determined as follow:
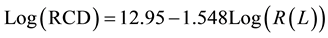
where R(L) is in kΩ and RCD in cm2. By following, the expected new radial column densities for the next two members of this oligomers in CW Leonis, CH3C9N and CH3C11N, should be expected near to 3.52 × 1010 [cm2] and 1.82 × 1010 [cm2], respectively.
4. Conclusions
Our results show a new route of linear oligomeric species analysis presenting in the ISM. Furthermore, the methodology developed from inner resistance to the dipole moments in these molecular wire systems opens new lines of research in the astronomical distribution and detection limits of these specific chemicals.
Particularly, in the present work, we show how dipole moments of these oligomeric series increase with chain length up to reach a certain saturation point, as can be seen in cyanopolyynes [19] and methylcyanopolyynes (this work), where R-[C≡C]14-CN establishes a molecular limit in every series. Therefore, we have developed a simple approach extendable to other similar molecular series.
Furthermore, our results and methodology on these molecular wires determine new tools of analysis to be
![]()
Figure 2. Observed trend between Radial Column Density (RCD) and Molecular Resistance (R(L)) in a logarithmic scale.
considered when chemical models are being used in a complex network of reaction schemes under LTE as well as in radial column density estimations of ISM. Effectively, our predictions about how the radial column density of long molecular wires declines with increasing molecular resistance, introduce a new criterion for radio searches of long molecules. The last question is important when a large amount of integration time will be necessary for observing these particular molecular systems.
Other linear molecular wires detected in circumstellar envelopes surrounding carbon-rich stars are being analyzed in our laboratory.
Acknowledgements
The authors acknowledge to the Centre for Environmental Sciences of the University of Chile for financial support.