1. Introduction
The squamates are the most diverse group containing the lizards and snakes [1] . Several investigations have been recorded on the fauna of Egyptian reptiles [2] [3] . The suborder Serpentes is distributed all over the world [4] [5] . Previous descriptions of the external and taxonomical features of some snakes have been ambiguous and unreliable. Therefore, several authors used the biochemical electrophoresis [6] and molecular sequence analysis [3] [7] - [13] to resolve the cladistic relationships among snakes and to clarify their phylogeny.
The family, viperidae comprises approximately 270 species of venomous snakes. The saw-scaled viper, Echis pyramidum (Geoffroy 1827) belongs to the family viperidae and is found in Asia, Africa, India, Iraq, Iran and Afghanistan. It occurs on a range of different substrates, including sand, rock, and soft soil and in scrublands, often found hiding under loose rocks [14] . Boidae, is a family of non-venomous snakes found in America, Africa, Europe, Asia, and some pacific islands. It includes relatively primitive snakes and comprises eight genera and 43 species. The kenyan sand boa, Eryx colubrinus (Linnaeus 1758) is found in northern Africa from Egypt as far west as Niger including Somalia, Ethiopia, Sudan, Kenya, and northern Tanzania [15] . It occurs in semi- desert, scrub savannahs and rock outcroppings. Also it prefers sandy and friable soil [16] . Extensive molecular genetic diversity has been discovered within and among populations and species in total protein [17] , isoenzymes/alloenzymes [18] and DNA [19] .
Isoenzymes are multiple forms of a single enzyme, which often have different isoelectric points and therefore can be separated by electrophoresis. Lactate dehydrogenases (Ldhs) isoenzymes are very suitable systems for studying several metabolic, genetic, ecological features, and are very useful in systematic studies [20] . Ldhs are a hydrogen transfer enzyme that catalyzes the oxidation of L-lactate to pyruvate with nicotinamide-adenine dinucleotide (NAD)+ as hydrogen acceptor, the final step in the metabolic chain of anaerobic glycolysis. Esterase isoenzymes (Est) are one of the lipid-hydrolyzing enzymes, possessing high significance in genetics and toxicology [21] .
Our present study aimed to investigate the patterns of inter-specific biochemical and genetic diversity between Echis pyramidum and Eryx colubrinus inhabiting two different sites in El-Faiyum desert of Egypt using the electrophoretic analyses of two isoenzymes.
2. Materials and Methods
2.1. Taxon Sampling and Study Area
A total of 10 individuals of two common Egyptian snake species were collected from two different sites in El- Faiyum desert (5 samples for each species). The first species was the saw-scaled viper, Echis pyramidum (Geoffroy 1827) (Reptilia: Viperidae). It is a medium sized snake with a short stocky body, moderate eyes separated from supralabials by scales and gray dorsum with a mid-dorsal series of dark-edged with whitish narrow-like marks [22] . It was collected from Gabal El-Nagar (Mahatet El-Rafa) [29˚22'N 30˚37'E]; a rocky area near Water drainage and cultivated area planted with wheat (Figure 1).
The other one was the Kenyan sand boa, Eryx colubrinus (Linnaeus 1758) (Reptilia: Boidae). It is a short thick snake with a relatively short tail end with a conical scale. It has also scales around the eyes, sandy dorsum with large irregularly shaped dark-brown blotches and yellowish ventral plain [22] . It was collected from Abshowy Kahek [29˚24'N 30˚38'E]; an area planted near a sandy road (Figure 1).
![]()
Figure 1. Photos of Echis pyramidum (a) and Eryx colubrinus (b) inhabiting El-Faiyum desert, Egypt.
2.2. Sample Preparation and Isoenzyme Assay
Tissue samples of liver and heart were removed and immediately taken to the lab and stored at −80˚C for further laboratory use. For isoenzyme extraction, approximately 0.5 g of tissue was homogenized in 10 mL saline solution (PBS, pH = 6.8) using a manual Homogenizer. The homogenates were centrifuged at 5000 rpm for 10 minutes and the supernatants were kept at −20˚C until use. The enzymes; alfa-esterase (α-Est) in heart and Lactate dehydrogenase (Ldh) in liver supernatants were separated by discontinuous polyacrylamide gel electrophoresis [23] [24] .
Electrophoresis was carried out conveniently in discontinuous polyacrylamide gels. An amount of 50 μl of the clear supernatant of the liver and muscle homogenate of each sample was mixed with 20 μl of protein dye (1% bromophenol blue) and 20 μl of 2% sucrose. 30 μl of the mixture per gel slot were used to be applied per each sample for isoenzymes electrophoresis. After electrophoresis, the gel was transferred into a staining solution (50 - 70 ml) according to [25] which was then replaced by a destaining mixture of methanol, acetic acid and water (5:1:5 v/v/v). A potential gradient of high voltage electrode [(20 v/cm), anode] across the gel was applied for 4 h at 8˚C for separation of the enzymes.
2.2.1. Ldh Isoenzyme
For Ldh and after electrophoresis, the gel was soaked in 100 mL of 0.2 M Tris-HCl (pH 8.0) containing 30 mg NBT, 25 mg EDTA, 50 mg NAD, 10 mg L-lactic acid and 2 mg PMS. 0.05 M Tris-HCl pH 8.5 was prepared by dissolving 0.605 g Tris in 50 mL distilled water. The pH was adjusted to 8.5 by HCl. Then the solution was completed to 100 ml by distilled water [26] .
2.2.2. α-EST Isoenzyme
Regarding α-Est, after electrophoresis, the gel was soaked in 0.5 M borate buffer (pH 4.1) for 90 minutes at 4˚C. This procedure lowers the pH of the gel from 8.8 to about 7 at which the reaction proceeds readily. The low temperature minimizes diffusion of the protein within the gel. The gel then was rinsed rapidly in two changes of double distilled water. The gel was stained for esterase activity by incubation at 37˚C in a substrate solution of 100 mg α-naphthyl acetate and 100 mg fast blue RR salt in 200 ml of 0.1 M phosphate buffer pH 6.5 [27] .
After the appearance of the enzyme bands, the reaction was stopped by washing the gel two or three times with tap water. This was followed by adding the fixative solution, which consists of ethanol and 20% glacial acetic acid (9:11 v/v). The gel was kept in the fixative solution for 24 hours and then was photographed.
All gels were scanned using Gel Doc-2001 Bio-Rad system. For isoenzymes, the bands of enzyme activity were designated according to the system nomenclature proposed by [28] . An abbreviation which corresponds to the name of the isoenzyme designated each locus. When multiple loci were involved, the fastest anodal protein band was designated as locus one, the next as locus two and so on.
2.3. Metabolic Study
Immediately after collection, snakes were dissected. Pieces of liver and thigh muscles were removed and immediately weighed in grams (g) to the nearest 0.01 g. They were stored frozen at −20˚C till use. Livers and thigh muscles were processed for estimation of total lipids and total protein according to the method of [29] and [30] , respectively using a kit of Biodiagnostics Company.
Our Institutional Animal Care and Use Committee (IACUC) at Zoology Department, Faculty of Science, Cairo University has approved this study protocol from the ethical point of view and according to Animal welfare Act of the Ministry of Agriculture in Egypt that enforces the humane treatment of animals.
3. Statistical Analysis
Student t-test in the PASW package v. 20 was used to calculate the significance difference of total lipids and total proteins within and between the snake species.
4. Results and Discussion
Ldhs isoenzymes are very suitable systems for studying several metabolic, genetic, ecological features, and are very useful in systematic studies [20] [31] . Ldhs are a hydrogen transfer enzymes that catalyze the oxidation of L-lactate to pyruvate with nicotinamide-adenine dinucleotide (NAD)+ as hydrogen acceptor, the final step in the metabolic chain of anaerobic glycolysis. The reaction is reversible and the reaction equilibrium strongly favours the reverse reaction, namely the reduction of pyruvate (P) to lactate (L):
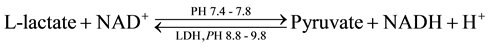
Three Ldhs isozymic forms were revealed in the liver tissues of both Echis pyramidum and Eryx colubrinus. The activity of Ldh-1 isoform seemed to be higher in E. pyramidum than in E. colubrinus. Such higher activity was reflected in the thicker and denser bands as well as their higher relative front (RF) in E. pyramidum (Figure 2).
The apparent increase in the activity of Ldh in liver tissues of E. pyramidum, in the present study, could be supported by the highly significant increase in the total protein and the highly significant decrease in the total lipids in liver and muscle tissues of this species (Table 1) [31] . It is thus possibly reasonable to suppose that E. pyramidum is more active, energetic and adaptable in the desert habitat than E. colubrinus.
α-Est showed five isozymic forms fractions in E. pyramidum, while it showed only four isoforms in E. colubrinus. α-Est-1 was the first clear, dense and thick isoform in E. pyramidum but it was completely absent in E. colubrinus. While the second isoform was revealed in E. colubrinus, it recorded only in three samples of the other species. The other isoforms; α-Est-3, α-Est-4 and α-Est-5 were dense and thick by the same extent in both E. pyramidum and E. colubrinus and also their relative front was nearly close to each other (Figure 3). The present results revealed the higher activity of alfa-esterases in the examined heart tissues of E. pyramidum than E. colubrinus. Esterases are used as bio-indicators to measure the toxic potency of pesticide and heavy metal residues usually applied in the field [20] [21] . The presence of the highly active esterase isoform; α-Est-1 only in the heart tissue of E. pyramidum may reflect to some extent, the unsafety of the diet applied to this species of snake and its high ability to resist and accumulate the toxic residues in its body tissues than in E. colubrinus.
Table 1 recorded the mean and standard error values of total lipids and total protein in liver and muscle tissues of both E. pyramidum and E. colubrinus. While the total lipids showed a very highly significant increases
![]()
Figure 2. The electrophoretic profile of Ldh isoenzymes in liver tissues. Lanes are as follow: 1 - 5 (E. pyramidum), 6 - 10 (E. colubrinus).
![]()
Figure 3. The electrophoretic profile of α-Est isoenzymes in the studied heart tissues. Lanes are as follow: 1 - 5 (E. pyramidum), 6 - 10 (E. colubrinus).
![]()
Table 1. Comparison of total lipids and total proteins in liver and muscle tissues of E. pyramidum and E. colubrinus. Data are expressed as mean ± standard error. Number of individuals between parentheses.
**Highly significant at P < 0.01, ***Very highly significant at P < 0.001.
in liver (P < 0.001) and muscle (P < 0.001) tissues of E. colubrinus than in E. pyramidum, E. pyramidum recorded a very highly significant increases (P < 0.001) in the total protein in liver and muscle tissues than in E. colubrinus. Within each species, while total lipids and total protein were significantly higher in liver than in muscle tissues (P < 0.01, P < 0.001 respectively). In E. pyramidum, they revealed a very highly significant increase (P < 0.001) in liver than in muscle tissues in E. colubrinus.
5. Conclusion
In conclusion, E. pyramidum acquired high physiological performance and activity than E. colubrinus, where Ldh isoenzyme expression in the first species was higher than in the second. The accumulations of total lipids and total protein were also significantly higher in the first species than in the second. The present data also revealed a high activity of the esterase isoform, α-Est-1 only in the heart tissue of E. pyramidum which may reflect the high ability of E. pyramidum to resist and accumulate the toxic residues in its body tissues than in E. colubrinus.
Acknowledgements
We are grateful to Prof. Dr. Hany Hassan, professor at Animal Reproduction Research Institute for his technical support in conducting the practical part of isoenzyme assay in this work.
NOTES
*Corresponding author.