Mathematical Modeling of Cardiomyocytes’ and Skeletal Muscle Fibers’ Membrane: Interaction with External Mechanical Field ()
1. Introduction
Every mechanical system, including living cells, in an external mechanical field is exposed to forces intrinsic to this field. The action of these forces results in mechanical tension that appears in cells. An external influence change (in direction or magnitude) leads to mechanical tension changes in cells and to deformation. The significance of the deformation for the cell depends on its inherent mechanical characteristics and the sensitivity of its mechanosensors.
An external physical signal transformation results in proper cell response generation. At the same time, the clue is the magnitude of the applied force that is able to induce cell response.
All living cells can be divided into two groups: cells that form internal tension only against external influence and cells that can additionally generate force themselves —muscle cells. Muscle cells have a specific structure, a developed cytoskeleton, which occupies most of the cell volume and forms a contractile apparatus. Taking these features into account, one can suppose that a muscle cell mechanosensor is connected to the contractile apparatus, for example with the M-line [1].
However, the muscle cell submembrane cytoskeleton is quite similar to that in non-muscle cells except for certain areas (particularly in areas of M-line and Z-disk projections on the membrane). Hence, cell formation takes place under constant external force action, so one can assume that the first mechanoreception acts to connect with the cell compartment, typical for every living cell. This compartment appears to comprise a membrane and a cortical (submembrane) cytoskeleton. Therefore, the question is what deformations emerge in the muscle fiber membrane after the gravity vector or the fiber contraction rate changes and whether these changes can result in muscle fiber mechanical characteristic change and cell response initiation.
To answer these questions, we need a numerical evaluation of deformations that arise in the sarcolemma after external mechanical conditions change. Such an evaluation requires data about longitudinal and transversal stiffness because muscle cells appear to have a three-dimensional structure [2]. However, this problem turns out to be hard to solve because the contractile apparatus contribution to linear stiffness is several orders greater than the contribution of the sarcolemma. Previously, we succeeded in determining the transversal stiffness of the sarcolemma using atomic force microscopy [3], changing mechanical conditions for both skeletal and cardiac muscles in rodents (Figure 1) [4].
An external mechanical condition change was implemented through the common animal antiorthostatic suspension method by tail at an angle of 30˚ respective to the cage floor (the Ilyin-Novikov method with the MoreyHolton modification is widely used in space physiology to model microgravity effects on a surface [5]). Animal suspension resulted, on the one hand, in a reduction of the external mechanical field on the hind limbs and, on the other hand, in increased mechanical tension in cardiomyocytes. The orientation of the muscle cells (muscle fibers) in the gravity field changed, too.
Nevertheless, the data for only the transversal stiffness of the membrane and cortical cytoskeleton do not afford us the influence of the gravity vector change on the muscle cell membrane and its probable involvement in primary mechanoreception acts. All of the above indicates the necessity of developing a membrane mathematical model.
2. Mathematical Model
2.1. Statement of the Problem
Let δ define the thickness of the membrane and cortical cytoskeleton, d is the muscle fiber diameter, and l is its length. Then, we can write the following relations:
 ,
, 
This enables us to consider the fiber a long, cylindrical envelope called a thin-walled rod [6].
It is typical for transversal sections of this kind of objects to initially be plane distorted on a surface: W(x, z). W(x, z) is usually called a sectional warping. For closed envelope rods, including muscle fibers, axial uniform warping is also typical, so we can rewrite W(x, z) as W(x). We have 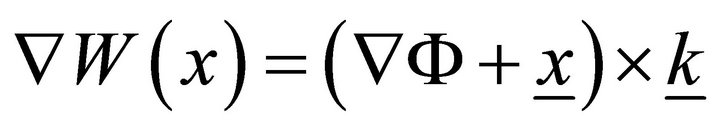 in Saint-Venant’s prob-
in Saint-Venant’s prob-

Figure 1. Scheme of muscle fiber with indicated measurement points. Using the AFM surface images, we were able to measure the transverse stiffness of all specific regions of the fiber, such as the contractile apparatus and membrane at different points. For this study, the main parameter is the membrane stiffness between the Z-disk and M-line projections. The structure of this region is universal for all cell types (not just muscle cells).
lem, where Ф is the Prandtl function (underlining indicates that the underlined value is a vector). We can take a sectorial area as W for a thin section. Then, for the part of membrane between the Z-disk and M-line to be considered, we use the thin-walled theory statements. We will consider the cylindrical rod with thin simply connected section F and with volume load f. For simplicity, we suppose the  end to be fixed, and we have the surface load
end to be fixed, and we have the surface load , which is a net load between the Zdisk and M-line (to be determined later) on the other end
, which is a net load between the Zdisk and M-line (to be determined later) on the other end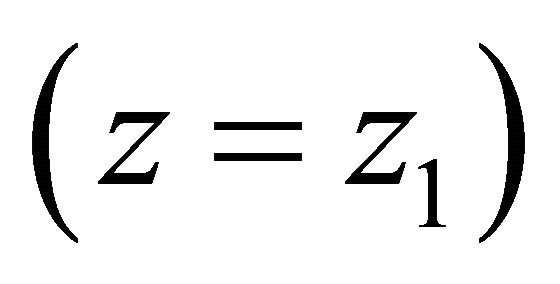 . We describe this three-dimensional case using the method of variations [7]:
. We describe this three-dimensional case using the method of variations [7]:
 , (1)
, (1)
 ,
,  where
where  is the translation vector,
is the translation vector,  is the volumetric energy,
is the volumetric energy,  is the Lamé constant,
is the Lamé constant, 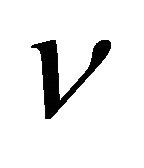 is the Poisson ratio,
is the Poisson ratio,  is the deformation tensor, and
is the deformation tensor, and  is the trace of deformation tensor, the first invariant (double underlining indicates tensors).
is the trace of deformation tensor, the first invariant (double underlining indicates tensors).
To derive equations from the variation principle, we approximate the translation as:
 , (2)
, (2)
where  is the linear translation,
is the linear translation, 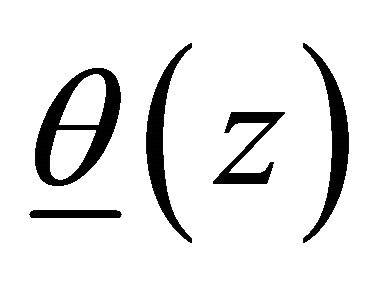 is the rotation vector,
is the rotation vector,  is the rotation angle per unit length, and
is the rotation angle per unit length, and  is the warping function:
is the warping function:
 ,
,  ,
,
 is the basis vector of the Lagrangian coordinate.
is the basis vector of the Lagrangian coordinate.
Let us suppose that there is a lack of transversal shifts; then:
 . (3)
. (3)
Taking into account Equation (3), approximation (2) becomes:
 , (4)
, (4)
That is:
 . (5)
. (5)
From the classical theory of elasticity, we know that:
 , (6)
, (6)
where S is the symmetrization symbol.
Using (4), Equation (6) can be rewritten:
 , (7)
, (7)
where  and
and  .
.
Then:
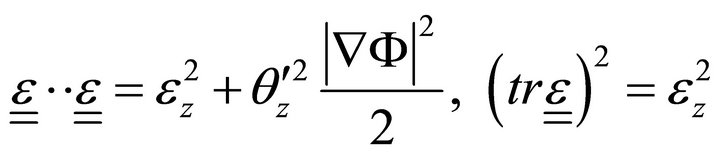 (8)
(8)
Substituting (8) into (1), we obtain a new volumetrical energy density:

Since  :
:
 , (9)
, (9)
where , and
, and  is Young’s modulus.
is Young’s modulus.
Taking into account (7), (9) becomes:
 . (10)
. (10)
Integrating (10) in the section:
 , (11)
, (11)
where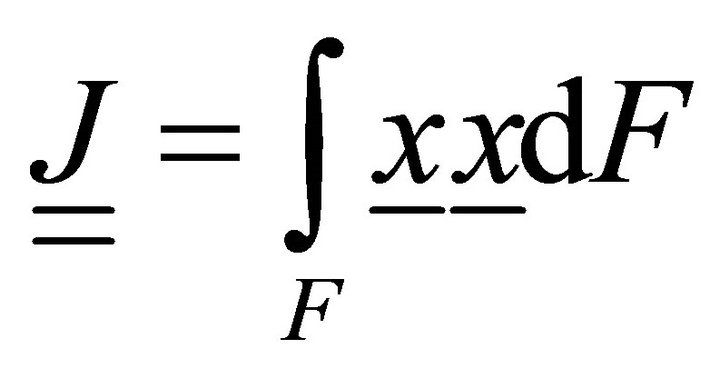 ,
,  ,
,  ,
,  ,
,  .
.
Let us now determine the work of volume loads:
 , (12)
, (12)
where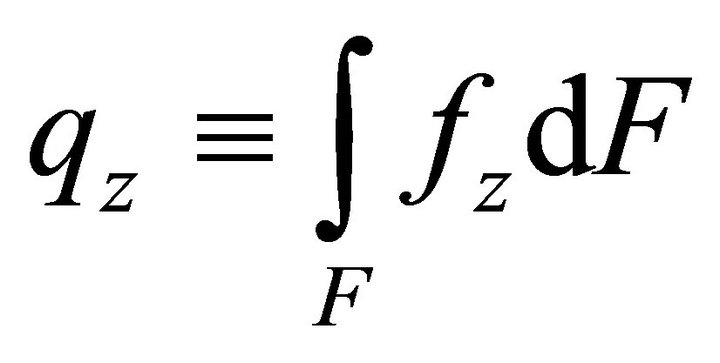 ,
,  ,
,  ,
, 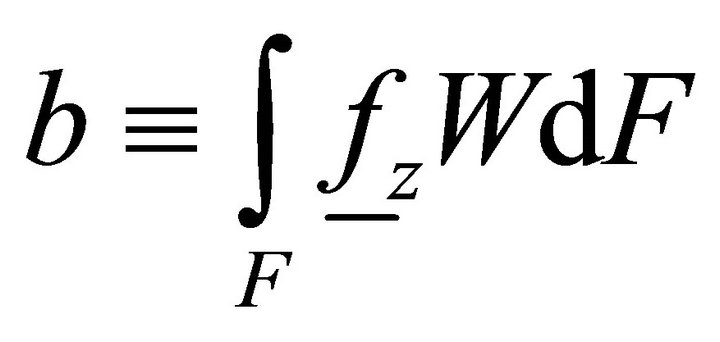 is the distributed bimoment per unit length.
is the distributed bimoment per unit length.
Similarly, a work of the volume load on the end:
 , (13)
, (13)
where ,
,  ,
,  ,
, 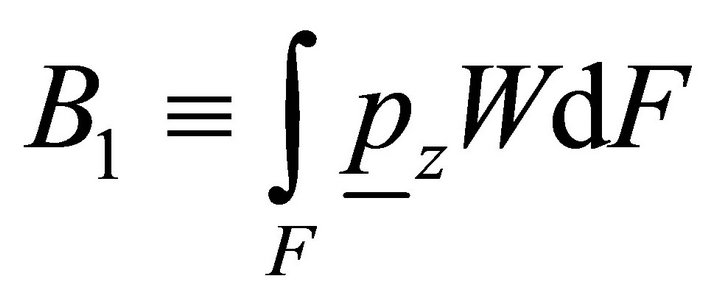 is the bimoment on the end.
is the bimoment on the end.
Taking into account Equations (12) and (13), the variational equation becomes:
 . (14)
. (14)
From (14), we obtain differential equations and limits:
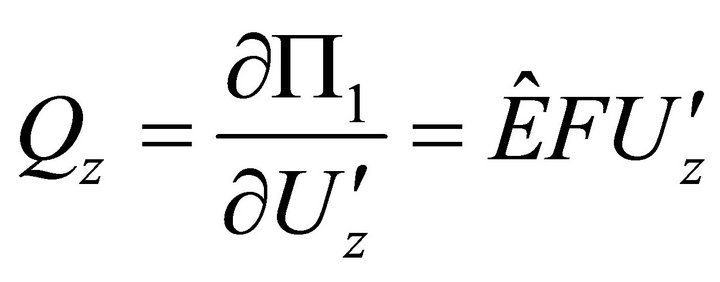
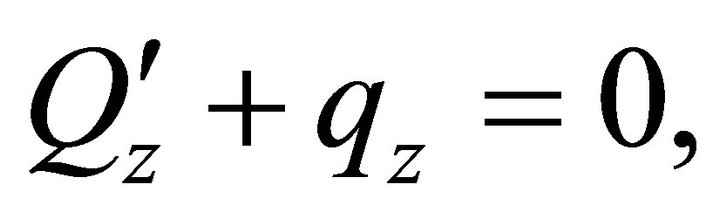 ,
,
 :
: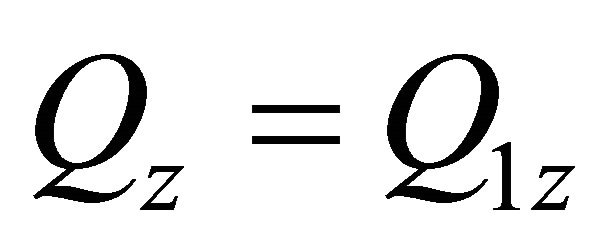 ,
,  ,
,  ,
,
 , (15)
, (15)
 :
: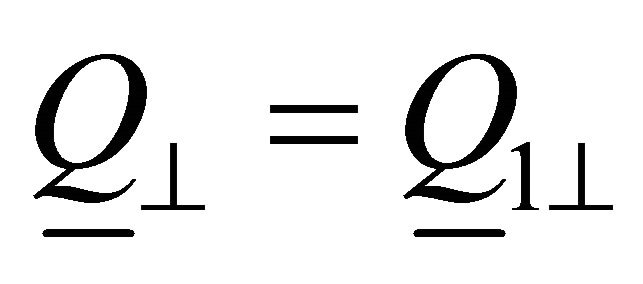 ,
, 
 ,
,  ,
,
 ,
,
 :
: ,
,  ,
, 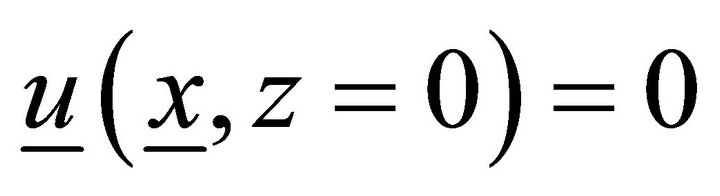
The set of equations derived in (15) gives us a chance to completely describe muscle fiber membrane behavior as a long cylindrical envelope and to find the potential energy  in the case described.
in the case described.
Let us assume that there is no external moment influence and that section warping and transversal shift contribution are negligible in comparison with the longitudinal component. This assumption is justified because of the specific muscle cell structure. Then, solving (15), we can find :
:
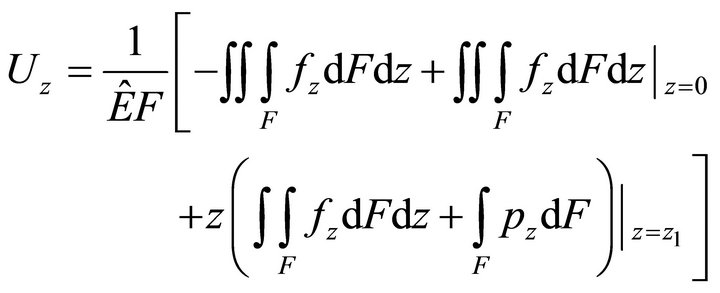 , (16)
, (16)
where  is the volume load,
is the volume load, 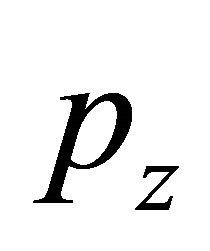 is the surface load, F is the section area,
is the surface load, F is the section area,  is the adduced Young’s modulus, and
is the adduced Young’s modulus, and  is the Poisson ratio.
is the Poisson ratio.
2.2. External Mechanical Loading
An external mechanical field acts on the whole organism and launches a number of processes, leading to nervous activation change in skeletal muscles, a liquid shift in the cranial direction, and, as a result, to a volume load change in the heart.
These processes results in the muscle fiber membrane becoming subjected to the following forces: 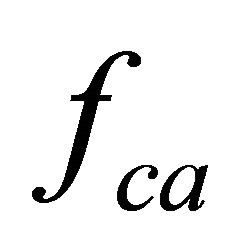 by the contractile apparatus as a result of nervous activation,
by the contractile apparatus as a result of nervous activation,  , hydrostatic pressure (only for cardiomyocytes), and
, hydrostatic pressure (only for cardiomyocytes), and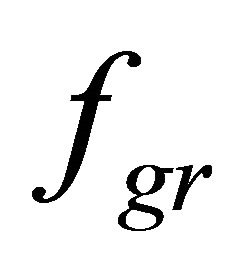 , the gravity. Nervous activation by intracellular signal mechanisms launch results in mechanical tension that arises in a muscle fiber because of myosin head and actin filament interaction, which is transmitted into the sarcolemma by the cortical cytoskeleton. Let us suppose that this interaction is uniform distributed over the length of the contractile apparatus. Therefore, it can be represented as a periodical function with the period of Т, which equals the distance between two successive myosin heads.
, the gravity. Nervous activation by intracellular signal mechanisms launch results in mechanical tension that arises in a muscle fiber because of myosin head and actin filament interaction, which is transmitted into the sarcolemma by the cortical cytoskeleton. Let us suppose that this interaction is uniform distributed over the length of the contractile apparatus. Therefore, it can be represented as a periodical function with the period of Т, which equals the distance between two successive myosin heads.
Then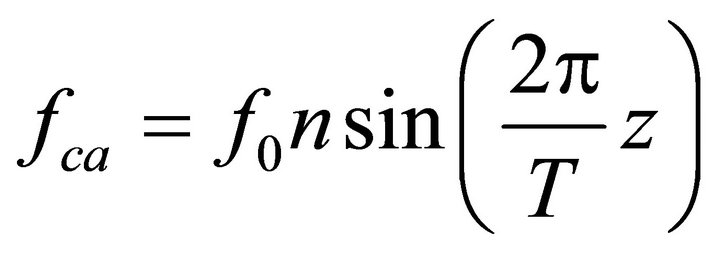 , where
, where 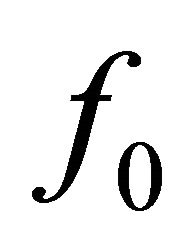 is the force generated by the single bridge, approximately 3 - 5 pN, n represents a number of bridges, which can be determined as the ratio of the fiber length (l) to the distance between two successive bridges, approximately 43 nm, per fiber volume. Gravity also acts on a muscle cell depending on the cell orientation. The specific volumetric force in this case may be represented as
is the force generated by the single bridge, approximately 3 - 5 pN, n represents a number of bridges, which can be determined as the ratio of the fiber length (l) to the distance between two successive bridges, approximately 43 nm, per fiber volume. Gravity also acts on a muscle cell depending on the cell orientation. The specific volumetric force in this case may be represented as , where
, where
 is the angle between the gravity vector and the fiber longitudinal axis direction,
is the angle between the gravity vector and the fiber longitudinal axis direction,  is the free-fall acceleration, and
is the free-fall acceleration, and 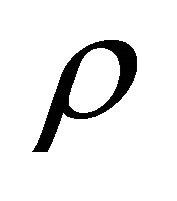 is the liquid density. The surface load on the end is a net load of gravity and hydrostatic pressure;
is the liquid density. The surface load on the end is a net load of gravity and hydrostatic pressure; , where d is the fiber diameter.
, where d is the fiber diameter.
Then, the external forces become:
 ,
,
 . (17)
. (17)
3. Numerical Examples
As an experimental model, we use a rodent’s antiorthostatic suspension (Figure 2).
We have determined in previous experiments Young’s modulus of the sarcolemma of different skeletal muscles [4] and a rat’s left ventricle [8,9]. Moreover, the introduction of the nifedipin system into rats resulted in increased sarcolemma transversal stiffness in skeletal muscles [10]. We have also determined the muscle fiber diameter. Based both on these data and ratio (16), we can find characteristic longitudinal deformations of M. soleus fibers and cardiomyocytes (Table 1).
4. Discussion and Conclusions
Interaction between a cell and an external mechanical field is still an unsolved problem in modern cell biophysics. A case of gravity vector change appears to be

Figure 2. The Ilyin-Novikov method with the Morey-Holton modification of a rodent’s antiorthostatic suspension [5]. The dotted arrows indicate the direction of muscle cells’ dominant axis (z axis) under normal conditions, and the solid arrows represent the suspension case.
Table 1. Longitudinal absolute and relative deformations of different muscle cells after antiorthostatic suspension at an angle of 30˚.