Preparation of Dithienylethene with Amino Groups, and Photochromic Behavior in Langmuir-Blodgett Film of the Amphiphilicdithienylethene ()
1. Introduction
Thermally irreversible (p-type) photochromic compounds are a promising candidate for optoelectronic devices, for example, optical memory [1], holography [2], photooptical switching [3], and display [4,5]. In particular, dithienylethenes are well-known to be very thermally stable in solution [6,7]. In addition, they have been reported to exhibit stable photochromism in crystal state because the change of their molecular structure is very small during photo chromic reaction, so that their photochromic reaction occurs even in crystal state [8]. Therefore, they are promising for the device applications.
For practical use as memory media, however, it is indispensable that the materials can form uniform film. Langumuir-Blodgett (LB) technique is a powerful tool to prepare uniform thin films whose film structure is controlled at a monomolecular level. If one can prepare LB film with multilayer structure consisting of different photo chromic compounds, multiple optical memory will be attained. Until now, few such studies have been conducted [9]. In these studies, however, they employed photochromic compounds which have large molecular structure change during their photochromic reaction, for example, azobenzene, spyropyran and so on [10-13]. The employment of the molecules with large molecular structure change during photochromic reaction is not appropriate to the application to rewritable optical memory since large molecular structure change disrupt the well-defined film structure. As a first step of aiming construction of multiple optical memory, in this study, we prepared LB film having dithienylethene chromophore, and evaluated its film structure and photochromic behavior. Here, we recited the reasons why we employed the thieny lethene chromophore as follows,
1) Dithienylethenes have small structure change during photochromic reaction; the photochromic reaction was observed even in their crystals [8,14];
2) They are thermally stable, in other words, they are not converted thermally to their initial non-coloured state after their conversion to coloured state by irradiation of UV light [15,16];
3) They have very fast response time of 2 - 3 ps for photochromic reaction [17];
4) They are also chemically stable; phochromic reaction cycle number is more than 104 [18,19].
In the previous work [20], S. Abe et al. demonstrated that the LB films of dithienylethene derivatives having long alky chain are successfully prepared when they are mixed with tripalmitin and that stable photochromic reaction in the LB films was confirmed by the change in fluorescence intensity caused by energy transfer from donor layer of a cyanine dye, which was deposited in the same LB films, to the dithienylethene layer when UV light and vis light were altermately irradiated. However, their LB films were mixed with other LB film forming amphiphile (tripalmitin and cadomium arachidate). Because the dithienylethene derivatives have weak hydrophilicity, in our consideration, the derivatives may require the mixing with the stable LB film forming amphiphiles for formation of stable Langmuir monolayer. The dilution of photochromoic spieces by mixing with inert LB film forming materials is not used to apply the LB film to high density optical memory. So that, by employing a photochromic amphiphile consisting of a cationic dithienylethene and anionic counter with long alkyl chain, we expected to form a stable LB film because of their balanced amphiphilicity for LB film formation. Further, if anionic counter ions with functional chromophore are employed, it is expected to construct multifunctional photochromic devices. In this paper, we report the LB film formation of the amphiphilic dithienylethene and its photochromic behavior.
2. Experiments
Figure 1 shows systhetic scheme of dithienylethene having ammonium bromide groups and molecular strcuture of dodecyl benzencsulfonic acid (DBS). The dithienylethene compoumd with ammonium group (DTE) and DBS were dissolved in chloroform (molar ratio; DTE: DOS = 1:2). By use of exstraction using H2O, then, unwished inorganic materials were pruned from the chloroform solution. Finally, dithienylethene having dodecyl benzenesulfonic acid as an anionic counter ion was obtained by evaporation of the solvent.
Non-coloured dithienylethene has two types of structure, i.e. anti-pallarel and pallarel. Since the pallarel structure does not exhibit photochromic reaction [17], the obtained dithienylethene having long dodecyl benzenesulfonic acid was sufficiently converted to its coloured species by irradiation of UV light passed through UV-pass filter (Siguma Koki, UTVAF-34U) from xenon lamp (Atago Bussan Co., LTD., XC-300) after dissolved in chloroform (conc. = 10−4 M); this means that we irradiated UV light until their absorption spectra were not changed. Monolayers of the dithienylethene were spread on a deionized water from the chloroform solution. The monolayers were deposited by the conventional Langmuir-Blodgett (LB) technique. The LB film preparation procedure was carried out in dark room. When the coloured structure in the obtained LB film were converted to non-color structure, we used visible light passed through sharp cut filter (Sigma Koki, SCF-50S-480Y) and IR cut filter (Sigma Koki, HAF-50S-15H) from the xenon lamp. For evaluation of reversible photochromic behavior in the LB films, above-mentioned the UV and visible light sources were employed.
3. Results and Discussion
Figure 2 shows surface pressure-area isotherm of the dithienylethene having long dodecyl benzenesulfonic acid as an anionic counter ion at 303 K on deionized water of > 1 MΩ·cm (MILLIPORE Milli-DI) as a subphase. The isotherm was measured using a coloured species which was converted by enough UV light irradiation. The isotherm exhibits very expanded region (from 1 nm2/molecules to 0.8 nm2/molecules) and through the plateau region, condensed region around 0.15 nm2/molecule is observed. We could not prepare LB film in the expanded region. In the condensed region, however, we
 (a)
(a) (b)
(b)
Figure 1. (a) Systhetic scheme of dithienylethene having ammonium bromide groups, and (b) molecular strcuture of dodecyl benzencsulfonic acid.
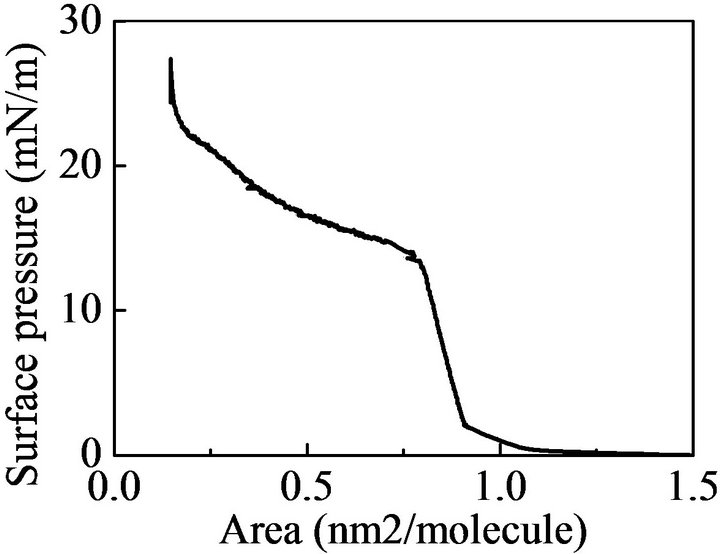
Figure 2. Surface pressure are isotherm of dithienylethene having long dodecyl benzenesulfonic acid as an anionic counter ion.
could prepare the LB films of dithienylethene having long dodecyl benzenesulfonic acid as an anionic counter ion.
Figure 3 shows X-ray diffraction profiles of as-deposited film, coloured film and non-coloured films. All X-ray diffraction profiles are very broadened, demonstrating the well-defined layered structure did not formed. From the broadened peaks, layer spacing of the LB films were estimated to be 12.4 nm. The value is very small in comparison of molecular length of dodecyl benzenesulfornic acid in which aklyl chain was assumed to form all zigzag trans-conformation. The result demonstrates that we did not obtain the LB film with well-defined layer structre; their orderliness is very low and they have a lot of defect as a result of a huge variety of alkyl chain conformation in the LB films.
Figure 4 (a) shows absorption spectra of as-deposited film, coloured film and non-coloured films. As-deposited films are peaking around 550 nm. Its colour was violet. By visible light irradiation, the as-depsited film was converted to non-coloured film. Further, by UV light irradiation, the non-coloured film was converted to coloured film. Unfortunately, reversibility of photochromic reaction in the LB films was not good; only several times photochromic reaction was observed in the LB films (Figure 4(b)). On the contrary, reversibility of thienylethene derivative and dodecyl benzenesulfonic acid, in which were dissolved, was very good. The absorbance is almost constant during the repetition photochromic reaction.
As the result of X-ray diffraction measurent, the LB films did not form well-defined layered structure and they have a lot of defect. Such not well-defined and lowdense structure facilitates penetration of oxygen gas into the LB films. The result suggests that disapperance of photochromic performance in the LB films [21] is most likely to be due to degradation of the dithienylethene by solfonic acid-catalyzed oxidization with oxygen, which penetrated into the LB film, dodecyl benzene solfonic acid has high acidity and because the photochromic reaction was carried out in the air. On the other hand, in the solution, the thienylethene molecules and dodecyl benzenesulfonic acid molecules are not so around each other;
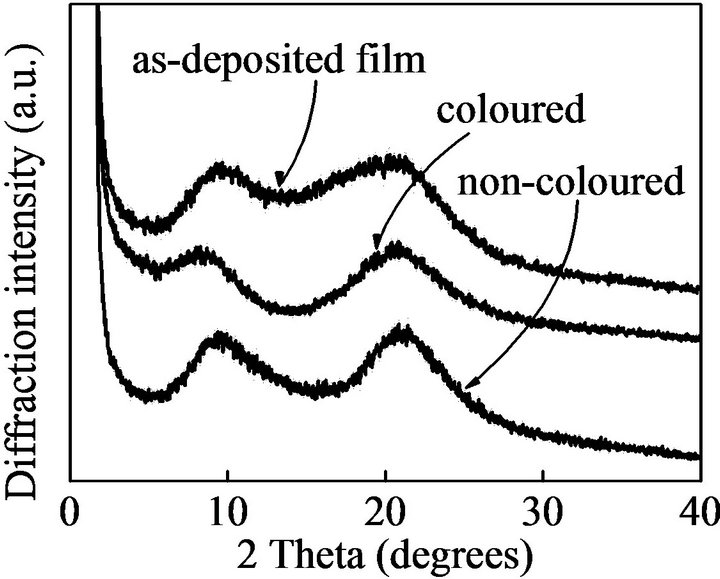
Figure 3. X-ray diffraction profiles of as-deposited film, coloured film and non-coloured films.
 (a)
(a) (b)
(b)
Figure 4. (a) Absorption spectra of as-deposited film, coloured film and non-coloured films, and (b) reversibility of the photochromic reaciton in the LB film (open circle): absorbance at 550 nm after irradiation UV light and fulled circle: Absorbance at 550 nm after irradiation UV light and that of the thienylethene derivative and dodecyl benzene solfonic acid in chroloform. The ordinate is normalized by absorbance of as-deposited LB film. In the case of solution, the ordinate is normalized by first coloured solution.
accordingly, the degradation as observed in the LB films was not ocuured.
4. Summary
In summary, we successfully prepared Langmuir-Blodgett (LB) films consisting of amphiphilic cationic dithienylethene by employing dodecyl benzenesulfonic acid as an anionic counter ion. Form X-ray diffraction profiles, however, it was demonstrated that they did not well defined layered structure. Photochromic behavior was confirmed in the LB films. However, the LB films were not so stable in the photochromic reaction; only several times photochromic reaction cycle was observed in the LB films.
For well-defined layered structre in LB film, we need to use counter anion having longer alkylchain. For the enhancement of the stability of photochromic behavior, we need to perform photochromic reaction in inert atmosphore or deposite passivation layer on the LB films to avoid penetration of oxygen; in S. Abe’s work in which they demonstrated stable photochromic reaction [20], their photochromic LB films were actually arachidic acid layerd which may play the role of passivation. In addition, the employment of week acidity counter anion with long alkyl chain such as arachidic adid is available to prevent acid-catalized degradation and enhance the photochromic performance.
NOTES