Flame Atomic Absorption Spectrometric Determination of Trace Amounts of Zinc and Thallium in Different Matrixes after Solid Phase Extraction on Modified Multiwalled Carbon Nanotubes ()
1. Introduction
The major increase in the use of toxic elements has resulted in high concentration of metals in aquatic systems. There are numerous sources of industrial effluents leading to heavy metal discharges apart from the mining and metal related industries [1-3]. Because of their toxicity and non biodegradable nature, metals are of special significance. The presence heavy metals in wastewater and surface water are becoming a severe environmental and public health problem.
Direct determination of metals in seawater samples by atomic absorption spectrometry, inductively coupled plasma optical emission spectrometry (ICP-OES) or inductively coupled plasma mass spectrometry (ICP-MS) is not always possible due to matrix interferences and the very low concentrations of metal ions [4].
Preconcentration is widely applied in a number method such as: flame atomic absorption spectro-metry (FAAS), ICP-OES, electrothermal atomic absorption spectrometry (ETAAS) or ICP-MS. In the first case, preconcentration step improves detection limit and enables analyte determination at trace concentrations [5]. The remaining methods are good analytical tools for trace analyses; nevertheless they are not free from influence of a complicated sample matrix. Therefore, apart from preconcentration, separation of an analyte from the matrix is frequently needed as it reduces interference effect by simplifying the sample matrix [6-9].
The most widely used techniques for the separation and preconcentration of trace metals include solid phase extraction, coprecipitation, liquid-liquid extraction, membrane filtration, floatation and cloud point extraction [10-18]. Among preconcentration methods, SPE has some advantages such as ease regeneration of solid phase, high preconcentration factor, and reusability of the adsorbent, low consumption of reagents, ease of automation, ecofriend methods and ease usage [19]. It is noticeable that SPE not on1y separates and concentrates samples but also performs as cleaner samples.
Since its discovery in 1991, carbon nanotubes (CNTs) have attracted great attention because of their unique properties. CNTs can be visualized as a sheet of graphite that has been rolled into a tube, and divided into multiwalled carbon nanotubes (MWNTs) [20] and singlewalled carbon nanotubes (SWNTs) [21] according to the carbon atom layers in the wall of the nanotubes. The hexagonal arrays of carbon atoms in graphene sheets of the CNTs surface have a strong interaction with other molecules or atoms, which make CNTs a promising adsorbent material as a replacement for activated carbon in many applications. Recently, CNTs show high efficiency for Pb2+, Cd2+ and F– removal from aqueous solution after oxidation treatment with nitric acid [22-24].
In the present work, the analytical potential of MWNTs modified with 1-(2-pyridylazo)-2-naphthol (PAN) as an adsorbent for the preconcentration of traces amounts of the zinc and thallium ions was investigated. This method is described in detail in the experimental section and was applied to measure zinc and thallium ions in water samples.
2. Experimental
2.1. Apparatus and Reagents
A Varian model SensAA GBC (Dandenong, Australia) atomic absorption spectrometer was used for measuring Tl(I) and Zn(II) in air-acetylene flame. A Metrohm pH meter (Herisau, Switzerland) was employed for pH measurements. A funnel-tipped glass tube (80 × 10 mm) was used as a column for preconcentration. All glass ware and columns were washed with a mixture of concentrated hydrochloric acid and concentrated nitric acid (1:1) before use. Deionized water was used for all dilutions. High purity reagents from Sigma (St. Louis, MO, USA) and Merck (Darmstadt, Germany) were used for all preparations of the standard and sample solutions. Stock solutions of thallium and zinc were prepared by dissolving appropriate amount of ultra pure salt obtained from Merck (Darmstadt, Germany) in 1 mol·L–1 HNO3. The working solutions were prepared by appropriate dilution of the stock solutions. Multiwalled carbon nanotubes of 95% purity and length 1 - 10 µm, number of walls 3 - 15 were purchased from Plasma Chem GmbH (Berlin, Germany). A 0.1% solution of 1-(2-pyridylazo)-2-naphthol (PAN) in ethanol was prepared. Buffer solution was prepared from 0.2 mol·L−1 potassium dihydrogen phosphate and disodium hydrogen phosphate for pH 6. Solutions of alkali metal salts (1%) and various metal salts (0.1%) were used for studying the interference of anions and cations, respectively.
2.2. Preparation of Column
A short glass column with a length of 80 mm and an inner diameter of 10 mm plugged with a small portion of cotton at both ends was prepared with fifty milligram of modified MWNTs. Before use, 5 mL of 2.0 mol·L–1 HCl solution and 10 mL of deionized were passed through the column in order to clean and condition it. Then, the column was conditioned to the described pH with 5 mL of buffer solution.
2.3. SPE Procedure
All standards and samples were prepared for analysis according to the following procedure. 20 mL of each sample was placed in a beaker. To each beaker, 5 mL of 0.2 mol·L−1 phosphate buffer (pH 6) was added. The resulting solution was passed through the column at flow rate of about 1.5 mL·min–1. Then, the column was rinsed with 5.0 mL of deionized and the adsorbed ions were eluted with 5.0 mL of 1.0 mol·L–1 HCl at flow rate of 0.8 mL·min–1. The final solution was aspirated directly into the flame atomic absorption spectrometry for determination of analyte ions.
3. Results and Discussion
A large number of studies have been reported on use of MWNTs for metal ion removal from aqueous solution but were not selective. Recent works [25-28] indicates that the MWCNTs can adsorb organic material, so we decided to add PAN to MWCNTs. These modified multiwalled carbon nanotubes have a greater capacity for adsorption and selectivity of ions.
The aim of the described research was to develop a sorbent for simultaneous separation and preconcentration of trace amounts of thallium and zinc. A simple, sensitive, rapid, and economical method was developed for flame atomic absorption spectrometry determination of trace amounts of thallium and zinc ions after separation and preconcentration by modified MWCNTs. Several factors that may affect the preconcentration and extraction process, including pH, type and volume of elution solution, flow rate of sample and eluent, sample volume and matrix effect were optimized.
3.1. Effect of the Sample pH
The formation of metal-chelate and its chemical stability are the two important influence factors for SPE. The influence of pH on the recovery of thallium and zinc ions was examined in the pH range of 3 - 9. The results are shown in Figure 1. The analyte ions were quantitatively recovered in the pH range of 3.0 - 6.5. At the pHs higher than 6.5 TlOH and Zn(OH)2 could be formed, therefore extraction efficiency was decreased. In subsequent experiments, the pH was kept at approximately 6 using potassium dihydrogen phosphate and disodium hydrogen phosphate buffer solution.
3.2. Effect of Eluent Type, Concentration and Volume
The selection of an appropriate eluent solvent is one of

Figure 1. The influence of the pH of aqueous solution on the recovery of thallium and zinc ions. Conditions: thallium, 25.0 µg; zinc, 1.0 µg; flow rate of sample, 1.5 mL·min–1; flow rate of eluent, 0.8 mL·min–1; final solution, 5.0 mL of 1.0 mol·L–1 HCl solution; reference, reagent blank.
the important factors to be considered in SPE. The eluent solvent should fulfill the following requirements: 1) the eluent should desorb the metals complexes; 2) the eluent should not destroy the sorbent; and 3) the eluent should be suitable for the subsequent determination technique [29].
Various inorganic and organic solvents (5.0 mL) were tested to choose the best solution for the elution of the analyte ions accumulated on modified MWCNTs, and the percentage recovery for each eluent type was determined. Organic solvents can be used as eluent but removed the complexing agent from the sorbent. If acid solutions were used as eluent, the reagent was retained on the sorbent and therefore, allowed using the column several times. Among the inorganic solvents studied, acids provided higher recovery efficiency compared to the other inorganic solvents, and the highest recoveries were obtained with HCl. The results are given in Table 1.
After this, the experiments were carried out for selecting the concentration of hydrochloric acid solution. Hydrochloric acid solutions at the concentrations of 0.5, 1.0, 1.5, 2.0 and 3.0 mol·L–1 were studied for this purpose.
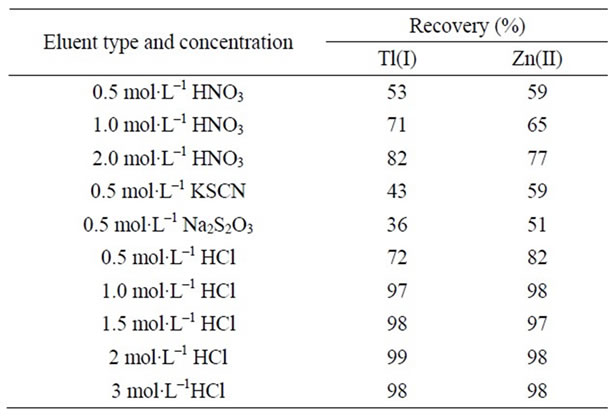
Table 1. Effect of type and concentration of eluent on the recovery of the analyte ions.
The results are given in Table 1. The results showed that the extraction efficiency increased with increasing concentration of hydrochloric acid solution up to 1.0 mol·L–1 and further concentration of hydrochloric acid solution has no effect on the recovery percent. Therefore, HCl 1.0 mol·L–1 solution was selected for further experiments.
For further enhancing the sensitivity and the preconcentration factor of the method low volume of eluent was used. For this purpose, the effect of eluent volume on the desorption of the analytes ions at a concentration of 1.0 mol·L–1 has been studied by keeping eluent concentration of 1.0 mol·L–1 and varying its volumes from 1.0 to 10.0 mL. The experimental results indicated that with 5.0 mL HCl 1.0 mol·L–1, quantitative recoveries (>97%) for the analyte ions could be obtained. Therefore, 5.0 mL of eluent volume was selected for subsequent experiments.
3.3. Effect of Sample Flow Rate
It is obvious that the flow rate of sample is an important parameter that controls the time of analysis and affects the retention of analyte ions on the sorbent. While the recovery increases with the decreasing flow rate, the preconcentration time increases. To obtain the quantitative recovery and to decrease the preconcentration time, the sample flow rate was investigated in the range 1 - 3 mL min·L–1. The results were shown that retention of the analyte ions were independent of flow rate in a range of 1 - 2 mL·min–1. Therefore a flow rate of 1.5 mL·min–1 was chosen for sample solutions in all subsequent experiments.
3.4. Effect of Eluent Flow Rate
The flow rate of eluent solution affects the recoveries of the analyte ions and duration of complete analysis. Therefore, the effect of the flow rates of eluent solutions were examined under the optimum conditions (pH and eluent type). The results were shown that desorption of the analyte ions were independent of flow rate in the range of 0.5 - 1.0 mL·min–1. Therefore a flow rate of 0.8 mL·min–1 was chosen for eluent solutions in all subsequent experiments.
3.5. Breakthrough Volume
The breakthrough volume is an important parameter in SPE because breakthrough volume represents the sample volume that can be preconcentrated without loss of analyte during elution of the sample [30]. The breakthrough volume of the sample solution was studied by dissolving 5.0 µg of thallium and 1.0 µg of zinc ions in different volumes (25 - 800 mL) and the SPE procedure was followed. The results were shown that, Tl(I) and Zn(II) ions were recovered quantitatively in the volume range of 25 - 700. Therefore, preconcentration factor (the ratio of the highest sample volume for both the analytes (700 mL) and the lowest eluent volume (5.0 mL)) was 140.
3.6. Adsorption Capacity
The adsorption capacity is the maximum metal quantity taken up by 1 g of modified sorbent and given by mg·g−1. To determine adsorption capacity of the modified MWNTs, a batch method was selected. 50 mL of aqueous solution containing 2.0 mg of thallium and 0.80 mg zinc at pH 6 was added to 0.1 g of modified MWNTs. After shaking for 10 min, the mixture was filtered and 10 mL of supernatant solution was diluted to 100.0 mL and was determined by FAAS. This procedure was repeated for each analyte ions separately. The capacity of modified MWNTs for thallium and zinc ions was found to be 8.8 and 6.4 mg·g–1, respectively.
3.7. Effect of Potentially Interfering Ions
In view of the high selectivity provided by flame atomic absorption spectrometry, the only interference may be attributed to the extraction step. Natural water samples contain commonly alkali, alkaline earth and some transition element salts. Therefore, the effects of some anions and cations at various concentrations on the recovery of the analyte ions were studied. For this purpose, an aliquot of aqueous solution (25 mL) containing 25.0 µg of thallium and 1.0 µg of zinc ions was taken with different amounts of foreign ions and the SPE procedure was followed. The tolerance limit was defined as the highest amount of foreign ions that produced an error no greater than ±5% in the determination of the analyte ions. The results are given in Table 2. The results demonstrate that the presence of large amounts of species commonly present in water samples have no significant effect on the determination of analyte ions.

Table 2. Tolerance limit of foreign ions.
3.8. Analytical Figures of Merite
Figures of merit of the method were obtained by processing standard solution of the analyte ions. Under the optimized conditions, calibration curves were constructed for the determination of thallium and zinc according to SPE procedure. Linearity was maintained between 0.1 to 20.0 mg·mL–1 for thallium and 20.0 ng·mL–1 to 0.5 mg·mL–1 for zinc in the final solution. The detection limits based on three times the standard deviation of the blank signal (n = 8) for thallium and zinc were 5.1 ng·mL–1 and 1.4 ng·mL–1, respectively. Seven replicate determination of a mixture of 5.0 mg·mL–1 thallium and 0.2 mg·mL–1 zinc in the final solution gave a mean absorbance of 0.085 and 0.074 with relative standard deviation 1.5% and 1.7%, respectively. The analytical parameters are given in Table 3.
3.9. Accuracy Check of the SPE Procedure
The accuracy of the SPE procedure was applied to the determination of thallium and zinc ions in National Institute for Environment Studies (NIES) No. 1 pepperbush and NIES No. 3 Chlorella. Approximately 0.01 g of NIES No. 1 and No. 3 were weighed accurately into a Teflon cup and dissolved in concentrated nitric acid (~10 mL) with heating on a water bath. The solution was cooled, diluted and filtered. The filtrate was made to 100.0 mL with deionized water in a calibrated flask. An aliquot of the sample solution was taken and thallium and zinc ions were determined by the SPE procedure. The results are given in Table 4, are in good agreement with the certified value.

Table 3. Analytical parameters of the method.
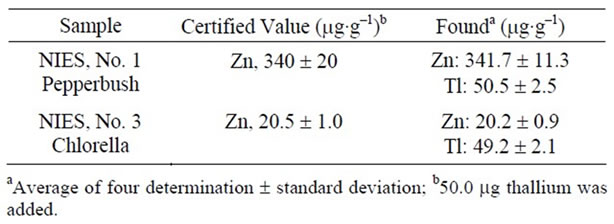
Table 4. Determination of Tl(I) and Zn(II) ions in certified reference materials.

Table 5. Determination of Tl(I) and Zn(II) ions in water samples.

Table 6. Comparison the detection limits of proposed methods with other methods.
3.10. Applicability of SPE Procedure for Analysis of Water Samples
River water samples were collected in acid-leached polyethylene bottles. The river water samples were collected from Rayen, Shahdad and Kohpayeh in Kerman Province, Iran. The only pretreatment was acidification to pH 2 with nitric acid, which was performed immediately after collection, in order to prevent adsorption of the metal ions on the flask walls. The samples were filtered before analyses through a cellulose membrane (Millipore) of 0.45 μm pore size.
The SPE procedure has been applied to the determination of thallium and zinc ions in different water samples. Also, the recovery of thallium and zinc ions from water samples spiked with Tl(I) and Zn(II) ions were studied. The results are given in Table 5.
The results were shown that, the added ions can be quantitatively recovered from the water samples by the SPE procedure. These results demonstrate the applicability of the SPE procedure for thallium and zinc ions determination in water samples.
4. Conclusion
The present study demonstrates preparation and use of a sorbent based on the modification of MWCNTs with PAN reagent. The modification of MWCNTs is simple, and the reagent remains in the column, which allows it to be used several times. It can be concluded from the results that modified MWNTs is an effective sorbent for trace amounts of thallium and zinc ions and can be used for its preconcentration from various samples. The proposed method has the following advantages: simple, rapid, high enrichment factor (140), reproducible, accurate and low analysis cost. Instead of the use of fresh solvent as an extracting phase for each sample, the reusability of adsorbent was as high as greater than 30 cycles without any loss in its sorption behavior. A comparison of the proposed method with the other reported preconcentration methods [31-39] for zinc and thallium extraction from water samples are given in Table 6. The obtained detection limits by the proposed method are comparable to most of those reported in the literature.