1. Introduction
Fused heterocyclic compounds are very important compounds partially because of their pharmacological properties which include wide applications in medicinal chemistry [1]. Nowadays, much attention has been drawn to pyrimidines and condensed pyrimidine compounds for their worthwhile and interesting biological properties [2].
Pyrimido[2,1-b]benzothiazole derivatives are evaluated for their High affinity central benzodiazepine recep tor ligands [3,4]. The pharmaceutical properties of these ligands range from anxiolytic/anticonvulsant for agonists to antigenic/convulsant for inverse agonists and it has been used for treating patients diagnosed with epilepsy. Additionally, these compounds are incorporated with pyrazole structure known to possess tranquilizing, psychoanalytic and muscle relaxant activities [5-7]. Pyrimidobenzothiazole derivatives have also been known for their antimicrobial properties [8-10], anti-allergy [11], antitumor and anti-viral activities [12]. Meanwhile, oxopyrimido benzothiazoles have been assessed for bronchodilators, and bronchial asthma treatment [13]. Besides, these types of compounds, especially those with amide groups, show incredibly potent anti-inflammatory, anticoagulant, anti-fungicidal and anti-herbicidal activities and are used in the chemotherapy of carcinoid patients [14-17].
Therefore, a variety of effective strategies have been developed for the synthesis of these compounds. Most of the methods depict synthesis of pyrimido[2,1-b]benzothiazole derivatives from 2-amino benzothiazoles and β-haloesters [8-10], orthoesters [14-17], allenic [18] and acetylenic groups [19-25]. Even though in some methods β-ketosters [26,27], α-haloacids [28] and malonates [29] have been used.
Although the above methods that have been described for the synthesis of pyrimido[2,1-b]benzothiazole derivatives have their own advantages, but many of these reported procedures lead to some disadvantages including low yields, prolonged reaction time, various use of reagents, catalysts, toxic organic solvents in the reaction media as well as high temperature during completion of the reaction. Recently, solvent free conditions has received considerable interest ascribed to increasing of Global concerns over harmful chemical reagents and replacement of noxious organic solvents is one of the most important goals in green chemistry.
In continuation of our research programs to find novel one-pot multicomponent reactions [30-35] herein, we wish to report the one-pot three component reaction of benzaldehyde derivatives, active methylene compounds and 2-amino benzothiazole in solvent-free conditions. (Scheme 1).
2. Results and Discussion
In an easy and expedient procedure, along with proper conditions, Benzaldehyde derivatives 1, β-ketosters 2, β- diketones 3, malonates 4 and 2-aminobenzothiazole 5 are used to synthesize 4H-pyrimido [2,1-b] benzothiazole and 2-oxo-pyrimido[2,1-b]benzothiazole compounds without using any solvent or catalyst. The corresponding products 6a-g has been achieved after 3 - 5 hr with good yields (Table 1).
We have introduced a one-pot three-component con-

Scheme 1. Synthesis of pyrimido[2,1-b]benzothiazole derivatives 6a-g.

Table 1. One-pot synthesis of pyrimido[2,1-b]benzothiazolesin the 60˚C in the solvent-free conditions*.
densation reaction (MCR) that is one of the most useful methods for the synthesis of organic compounds in an optimistic time and only in a single step. Moreover, the aforementioned reaction most often leads to product that can be easily separated and purified by simple filtering and washing out with a regular solvent. In fact, we have not established a mechanism for the formation of these two compounds although a proposed mechanism is indicated in Schemes 2 and 3.
It seems benzaldehyde as an electrophile and β-ketosters (2)/β-diketones (3), malonate (4) derivatives as active methylene compounds take part through an in-situ Knoevenagel reaction and an alkene is primarily formed. Afterwards, during the Michael addition reaction, 2- aminbenzothiazole as a Michael donor attacks alkene during nucleophilic reaction, so an iminium ion is formed, subsequently with a proton transformation and an intramolecular cyclization, products 6a-g are produced. In the final step, during intramolecular cyclization, malonates with two proper leaving groups (two alkoxy groups), with considering the temperature, easily omit carbon dioxide from their structure and give rise to the formation of compounds 6f-h (2-oxo-pyrimido[2,1-b]benzotiazole derivatives). Schemes 1 and 2 show the proposed mechanisms for the formation of products.
The structures of compounds 6a-g were deduced from their 1H NMR, 13C NMR and IR spectral data and also by mass spectrometry. The 6a-e products exhibited a singlet in 1H spectra at about δ = 5.54 - 6.33 ppm for H-4 and also a distinguished peak at δ = 50.9 - 56.5 ppm for C-4 in 13CNMR spectroscopy. The 6f-g products demonstrated a singlet in 1H spectra at δ = 9.02 - 10.2 ppm for H-3 and also a distinguished peak at δ = 110.9 - 116.2 ppm for C-3 in 13CNMR spectroscopy. The mass spectra of these compounds displayed molecular ion peaks. The selected spectroscopic data are reported in the experimental section.
3. Conclusion
As a result, 4H-pyrimido[2,1-b]benzothiazole (6a-e) and 2-oxo-pyrimido[2,1-b]benzothiazole (6f-g) are formed smoothly with the reaction of β-ketosters, β-diketone, malonates and benzaldehyde derivatives in the solventfree conditions with no solvent as well as no catalyst and subsequent annulation’s reactions proceeded in accept-

Scheme 2. Proposed mechanism for the synthesis of 4H-pyrimido[2,1-b]benzothiazole derivatives 6a-e in the solvent-free conditions.

Scheme 3. Proposed mechanism for the synthesis of 2-oxo-pyrimido[2,1-b]benzothiazole 6f-h derivatives in the solvent-free conditions.
able yields. These derivatives present a class of compounds that can be used as procedures for the synthesis of new derivatives with useful biological activities. In addition, our method has significant advantages, such as the high bond forming efficiency, solvent-free reaction conditions.
4. Experimental
4.1. Instruments and Characterization
Melting points were recorded on an Electrothermal 9100 melting point apparatus and Infrared (IR) spectra were recorded on a ABB FTLA-2000 spectrophotometer using KBr disks. 1H-NMR and 13C-NMR spectra were recorded on Bruker DRX 500 (500 MHz) AVANCE spectrometer in DMSO using TMS as the internal standard. Mass spectra were recorded on HP 5379(Agilent Technology) (EI, 20eV, 70eV).
4.2. General Procedure for the Synthesis of 4H-Pyrimido[2,1-b]benzothiazole-3-carboxylic Acid, 2-Methyl-4aryl-aklyl Ester:(6a-e)
A mixture of 2-aminobenzothiazole (1 mmol, 152 mg) and benzaldehyde derivatives (1 mmol) and ethylacetoacetate (1 mmol, 134 mg) or methylacetoactate (1 mmol, 125 mg) or acetylacetone (1 mmol, 97 mg) were heated at 60˚C in the solvent-free conditions for 4 - 5 hr. Completion of the reaction was confirmed by TLC (Petroleum ether:EtOAc 1:4). At the end of the reaction, the mixture was washed 3 times (3 ´ 20 ml) with water and diethylether. The desired products were obtained with high purity.
4.3. General Procedure for the Synthesis of 4-Aryl-2H-pyrimido-[2,1-b]benzothiazol-2-one: (6f-g)
A mixture of 2-aminobenzothiazole (1 mmol, 152 mg) and benzaldehyde derivatives (1 mmol) and diethylmalonate (1 mmol, 160 mg) or dimethylmalonate (1 mmol, 132 mg) was heated at 60˚C in the solvent-free conditions for 3 - 3.5 hr. Completion of the reaction was confirmed by TLC (Petroleum ether: EtOAc 1:2). At the end of the reaction the mixture was washed 2 - 3 times with water and diethylether. The desired products were obtained with high purity. The purity of prepared compounds was tested by the elemental analysis of C, H, and N elements using a Heraus CHN rapid analyzer.
4.4. Selected Data for Compounds 6a-g
4H-Pyrimido[2,1-b]benzothiazole-3-carboxylic acid-2- methyl-4(3-hydroxy phenyl)-methyl ester (6a, C19H- 16N2O2S):
218 mg (62%), M.p.: 283˚C - 284˚C; 1H NMR (500 MHz, DMSO-d6): δ = 2.29 (s, 3H, CH3), 3.61(s, 3H, CH3), 6.38 (s, 1H, CH), 6.58 (d, 1H, J = 10 Hz , HAr), 6.77 (s, H, HAr), 6.82 (d, 1H, J = 10 Hz, HAr), 7.05 (t, 1H, J = 13 Hz, HAr), 7.18 (t, 1H, J = 12 Hz, HAr), 7.28 (t, 1H, J = 13 Hz , HAr), 7.36 (d, 1H, J = 12.8 Hz, HAr), 7.73 (d, 1H, J = 12.8 Hz, HAr), 9.45 (s ,1H, OH)ppm; 13C NMR (125 MHz, DMSO-d6): δ = 23.3, 50.9 , 56.4, 102.6, 112.3 , 113.4, 115.3, 117.6, 122.8, 122.9, 124.1, 126.8, 129.5, 137.6, 142.8, 153.8, 154.1, 157.6, 162.8, 166.1 ppm; IR (KBr): 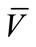 = 2974, 2608, 1694, 1607 cm‑1; MS (EI, 20 eV) C19H16N2O3S, 352 (78%, M+·), 337 (14%, [MMe]+), 321 (8%, [M-OMe]+), 293 (51%, [M-CO2Me]+), 259 (100%, [M-C6H5O]+),199 (45%, [M-C9H11O3]+); Anal. Calcd for C19H16N2O3S: C 64.76, H 4.58, N 7.95, Found C 64.60, H 4.43, N 7.85
= 2974, 2608, 1694, 1607 cm‑1; MS (EI, 20 eV) C19H16N2O3S, 352 (78%, M+·), 337 (14%, [MMe]+), 321 (8%, [M-OMe]+), 293 (51%, [M-CO2Me]+), 259 (100%, [M-C6H5O]+),199 (45%, [M-C9H11O3]+); Anal. Calcd for C19H16N2O3S: C 64.76, H 4.58, N 7.95, Found C 64.60, H 4.43, N 7.85
4H-pyrimido[2,1-b]benzothiazole-3-carboxylic acid-2- methyl-4(3-hydroxy phenyl)-ethyl ester (6b C20H18N2O3S):
234 mg (64%), Mp = 258˚C - 260˚C;1H NMR (500 MHz, DMSO-d6): δ = 1.22 (t, J = 7 Hz , 3H, CH3), 2.32 (s, 3H, CH3), 4.08 (m, 2H, J = 8 Hz, CH2), 6.39 (s, 1H, CH), 6.60 (d, 1H, J = 8 Hz, HAr), 6.80 (s,1H, HAr), 6.87 (d, 1H, J = 8 Hz, HAr), 7.07 (t, 1H, J = 8 Hz, HAr), 7.2 (t, 1H, J = 7.5 Hz, HAr), 7.32 (t, 1H, J = 7.5Hz, HAr), 7.38 (d, 1H, J = 8 Hz, HAr), 7.76 (d, 1H, J = 8 Hz, HAr), 9.45 (s ,1H, OH)ppm;13C NMR (125 MHz, DMSO-d6): δ = 14.1, 23.2, 56.5, 59.6, 102.8, 112,3, 113.6, 115.3, 117.7, 122.8, 124.0, 126.8, 129.4, 137.6, 142.8, 153.8, 157.5, 162.6, 166.6 ppm;IR (KBr):  = 2978, 2587, 1694, 1597 cm‑1; MS (EI, 20 eV) C20H18N2O3S, 366 (60%, M+·), 351 - 337 (25%, [M-Et]+·), 293 (53%, [M-CO2Et]+·), 273 (100%, [M-C6H5O]+·), Anal. Calcd for C20H18N2O3S: C 65.55, H 4.95, N 7.64, Found C 64.43, H 4.88, N 7.58.
= 2978, 2587, 1694, 1597 cm‑1; MS (EI, 20 eV) C20H18N2O3S, 366 (60%, M+·), 351 - 337 (25%, [M-Et]+·), 293 (53%, [M-CO2Et]+·), 273 (100%, [M-C6H5O]+·), Anal. Calcd for C20H18N2O3S: C 65.55, H 4.95, N 7.64, Found C 64.43, H 4.88, N 7.58.
4H-pyrimido[2,1-b]benzothiazole-3-carboxylic acid-2- methyl-4(3-nitro phenyl)-ethyl ester (6c, C20H17N3O4S)
237 mg (60%); Mp = 222˚C - 224˚C; 1H NMR (500 MHz, DMSO-d6): δ = 1.18 (t, 3H, J = 8.3 Hz, CH3), 2.33 (s, 3H, CH3), 4.05 (m, 2H, CH2), 6.69 (s, 1H, CH), 7.2 (t, 1H, J = 11.5 Hz, HAr), 7.30 (t, 1H, J = 11.5 Hz, HAr), 7.54 (d, 1H, J = 14.5 Hz, HAr), 7.59 (t, 1H, J = 13.3 Hz, HAr), 7.77 (d, 1H, J = 12 Hz , HAr), 7.85 (d, 1H, J = 13.5 Hz , HAr) 8.08 (d, 1H, J = 13.5 Hz, HAr), 8.34 (s, 1H, HAr); IR (KBr):  = 2962, 1689, 1607, 1494 cm‑1, Anal. Calcd for C20H17N3O4S: C 60.48, H 4.33, N 10.63, Found C 60.40, H 4.38, N 10.52.
= 2962, 1689, 1607, 1494 cm‑1, Anal. Calcd for C20H17N3O4S: C 60.48, H 4.33, N 10.63, Found C 60.40, H 4.38, N 10.52.
4H-pyrimido[2,1-b]benzothiazole-3-carboxylic acid-2- methyl-4(4-hydroxy phenyl)-ethyl ester(6d, C20H18N2O3S)
230 mg (63%); Mp = 267˚C - 269˚C;1H NMR (500 MHz, DMSO-d6): δ = 1.18 (t, 3H, J = 12 Hz, CH3), 2.3 (s, 3H, CH3), 4.03 (m, 2H, CH2), 6.33 (s, 1H, CH), 6.62 (d, 1H, J = 14 Hz, HAr), 7.15 (t, 1H, J = 12 Hz, HAr), 7.21 (t, 1H, J = 14 Hz, HAr), 7.28 (t, 1H, J = 12 Hz, HAr), 7.39 (d, 1H, J = 12.7 Hz, HAr), 7.71 (d, 1H, J = 12.7Hz, HAr), 9.46 (s, 1H, OH) pppm; 13C NMR (125 MHz, DMSO-d6): δ = 14.1, 23.2, 56.2 , 59.4, 103.1, 112.4, 115.1, 122.8, 122.8, 123.9, 126.7, 128.4, 132.2, 137.7, 153.6, 157.3, 162.4, 165.65 ppm; IR (KBr):  =2 947, 1674, 1591, 1514 cm‑1; MS (EI, 20 eV) C20H18N2O3S, 366 (47%, M+·), 337 (22%, [M-Et]+), 293 (100%, [M-CO2Et]+), 273 (86%, [M-C6H5O]+). Anal. Calcd for C20H18N2O3S: C 65.55, H 4.95, N 7.64, Found C 64.45, H 4.90, N 7.60.
=2 947, 1674, 1591, 1514 cm‑1; MS (EI, 20 eV) C20H18N2O3S, 366 (47%, M+·), 337 (22%, [M-Et]+), 293 (100%, [M-CO2Et]+), 273 (86%, [M-C6H5O]+). Anal. Calcd for C20H18N2O3S: C 65.55, H 4.95, N 7.64, Found C 64.45, H 4.90, N 7.60.
4H-pyrimido[2,1-b]benzothiazole-3-acetyl-2-methyl-4(3-hydroxyphenyl) (6e, C19H16N2O2S)
220 mg (60%); Mp = 290˚C - 292˚C;1H NMR (500 MHz, DMSO-d6): δ = 2.29 (s, 3H, CH3), 2.33 (s, 3H, CH3), 6.51 (s, 1H, CH), 6.57 (d, 1H, J = 16 Hz, HAr), 6.79 (t, 1H, J = 3.5 Hz , HAr), 6.85 (d, 1H, J = 13Hz, HAr), 7.03 (t, 1H, J = 13 Hz, HAr), 7.20 (t, 1H, J = 13 Hz, HAr), 7.34 (t, 1H, J = 13 Hz, HAr), 7.51 (d, 1H, J = 13 Hz, HAr), 7.75 (d, 1H, J = 13 Hz, HAr), 9.43 (s, 1H, OH).ppm, 13C NMR (125 MHz, DMSO-d6): δ = 13.1, 56.5, 59.2, 103.3, 112.3, 113.2, 115.7, 117.2, 121.8, 123.7, 126.8, 129.4, 136.3, 142.3, 153.7, 157.5, 161.4, 166.8, IR (KBr):  = 2931, 2608, 1617, 1590, 1499 cm‑1; MS (EI, 20eV) C19H16N2O2S, 336 (65%, M+), 293 (100%, [M-COMe]+), 243 (100%, [M-C6H5O]+). Anal. Calcd for C19H16N2O2S: C 67.83, H 4.79, N 8.33, Found C 67.71, H 4.73, N 8.26.
= 2931, 2608, 1617, 1590, 1499 cm‑1; MS (EI, 20eV) C19H16N2O2S, 336 (65%, M+), 293 (100%, [M-COMe]+), 243 (100%, [M-C6H5O]+). Anal. Calcd for C19H16N2O2S: C 67.83, H 4.79, N 8.33, Found C 67.71, H 4.73, N 8.26.
4-(4-hydroxyphenyl)-2H-pyrimido-[2,1-b]benzothiazol-2-one (6f, C16H10N2O2S)
202 mg (69%); Mp = 226˚C - 228˚C; 1H NMR (500 MHz, DMSO-d6): δ = 6.95 (d, 1H, J = 14.4 Hz, HAr), 7.35 (t, 1H, J = 13.4 Hz, HAr), 7.47 (t, 1H, J = 13.7 Hz, HAr), 7.87 (d, 1H, J = 13.2 Hz, HAr), 7.94 (d, 1H, J = 14.4 Hz, HAr), 8.01(d, 1H, J = 13.2 Hz, HAr), 9.02 (s, 1H, CH), 10.61 (s, 1H, OH).ppm; 13C NMR (125 MHz, DMSO-d6): δ = 116.2, 122.2, 122.3, 124.8, 125.9, 126.5, 132.7, 133.9, 151.4, 162.9, 166.4, 172.0 ppm; IR (KBr): 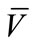 = 3024, 2603, 1607,1571, 1524 cm‑1; MS (EI, 70 eV) C16H10N2O2S, 294 (8%, M+·), 293 (4%, [M−1]+), 254 (89), 253 (100%). Anal. Calcd for C16H10N2O2S: C 65.29, H 3.42, N 9.51, Found C 65.20, H3.36, N 9.44.
= 3024, 2603, 1607,1571, 1524 cm‑1; MS (EI, 70 eV) C16H10N2O2S, 294 (8%, M+·), 293 (4%, [M−1]+), 254 (89), 253 (100%). Anal. Calcd for C16H10N2O2S: C 65.29, H 3.42, N 9.51, Found C 65.20, H3.36, N 9.44.
4-(2-hydroxy-5-bromo-phenyl)-2H-pyrimido-[2,1-b]benzothiazol-2-one (6g,C16H9BrN2O2S)
268 mg (72%); Mp = 173˚C - 174˚C; 1H NMR (500 MHz, DMSO-d6): δ = 6.98 (d, 1H, J = 14.7 Hz, HAr), 7.4 (t, 1H, J = 12 Hz, HAr), 7.5 (t, 1H, J = 12 Hz , HAr), 7.61 (d, 1H, J = 14.7 Hz, HAr), 7.93 (d, 1H, J = 13 Hz, HAr), 8.04 (d, 1H, J = 10.5 Hz, HAr), 8.05 (s, 1H, HAr) ppm; 13C NMR (125 MHz, DMSO-d6): δ = 110.9, 119.4, 121.7, 122.4, 122.7, 125.4, 126.8, 131.8, 134.1, 137.6, 151.2, 159.5, 163.6, 170.3 ppm; IR (KBr):  = 3100, 1606, 1565, 1493 cm‑1; MS (EI, 70 eV) C16H9N2O2SBr, 374 (M+, C16H9N2O2S81Br), 372 (M·+,C16H9N2O2S79Br), 335 (79), 334 (100), 333(79%). Anal. Calcd for C16H9N2O2SBr: C 51.63, H 2.44, N 7.52, Found C 51.55, H 2.39, N 7.46.
= 3100, 1606, 1565, 1493 cm‑1; MS (EI, 70 eV) C16H9N2O2SBr, 374 (M+, C16H9N2O2S81Br), 372 (M·+,C16H9N2O2S79Br), 335 (79), 334 (100), 333(79%). Anal. Calcd for C16H9N2O2SBr: C 51.63, H 2.44, N 7.52, Found C 51.55, H 2.39, N 7.46.
5. Acknowledgements
Saeed Balalaie thanks Iran National Science Foundations (INSF) for the financial support.
NOTES