I. Wacławska et al. / Natural Science 3 (2011) 689-693
Copyright © 2011 SciRes. OPEN ACCESS
692
(a)
(b)
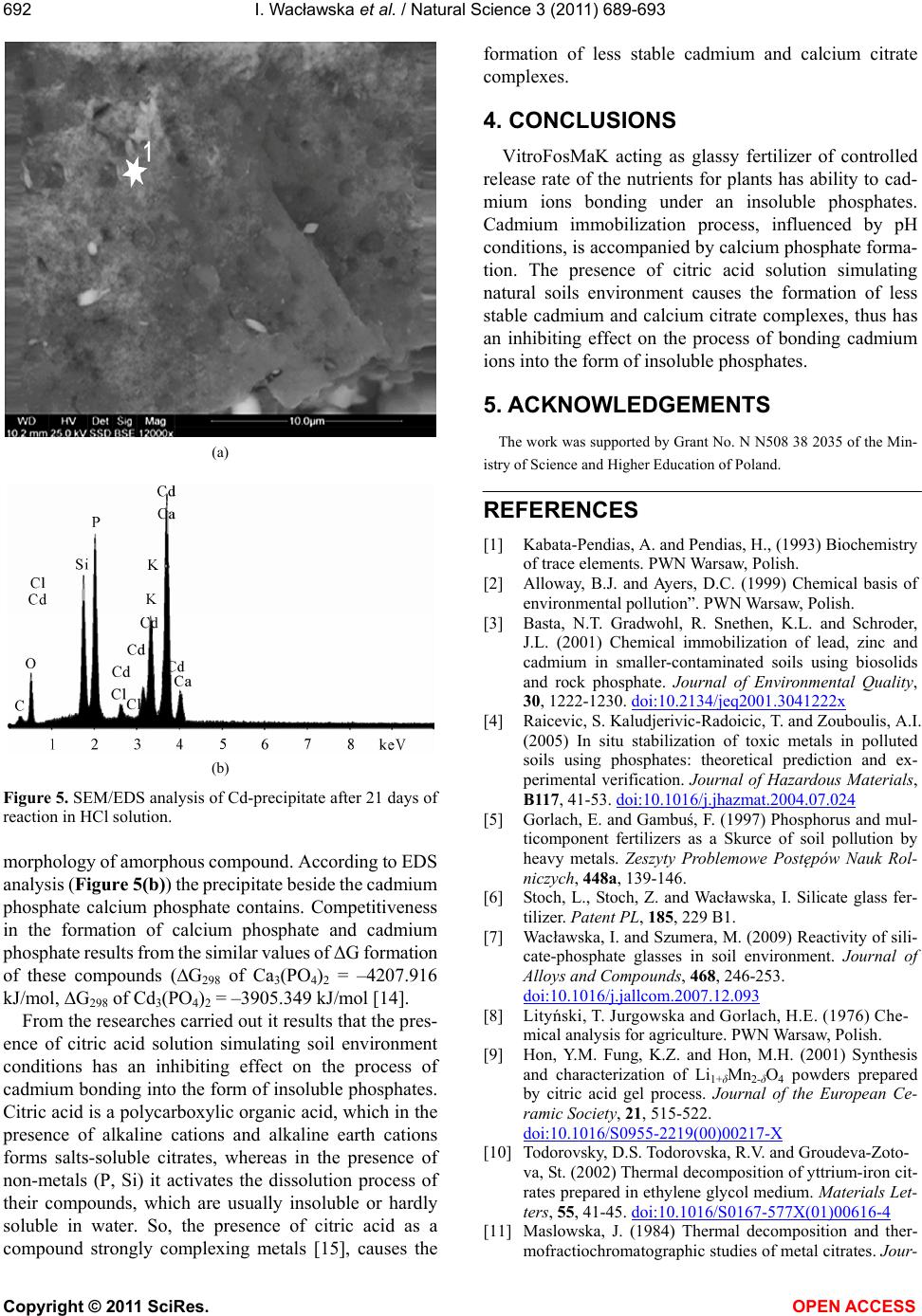
Figure 5. SEM/EDS analysis of Cd-precipitate after 21 days of
reaction in HCl solution.
morphology of amorphous compound. According to EDS
analysis (Figure 5(b)) the precipitate beside the cadmium
phosphate calcium phosphate contains. Competitiveness
in the formation of calcium phosphate and cadmium
phosphate results from the similar values of ΔG formation
of these compounds (∆G298 of Ca3(PO4)2 = –4207.916
kJ/mol, ∆G298 of Cd3(PO4)2 = –3905.349 kJ/mol [14].
From the researches carried out it results that the pres-
ence of citric acid solution simulating soil environment
conditions has an inhibiting effect on the process of
cadmium bonding into the form of insoluble phosphates.
Citric acid is a polycarboxylic organic acid, which in the
presence of alkaline cations and alkaline earth cations
forms salts-soluble citrates, whereas in the presence of
non-metals (P, Si) it activates the dissolution process of
their compounds, which are usually insoluble or hardly
soluble in water. So, the presence of citric acid as a
compound strongly complexing metals [15], causes the
formation of less stable cadmium and calcium citrate
complexes.
4. CONCLUSIONS
VitroFosMaK acting as glassy fertilizer of controlled
release rate of the nutrients for plants has ability to cad-
mium ions bonding under an insoluble phosphates.
Cadmium immobilization process, influenced by pH
conditions, is accompanied by calcium phosphate forma-
tion. The presence of citric acid solution simulating
natural soils environment causes the formation of less
stable cadmium and calcium citrate complexes, thus has
an inhibiting effect on the process of bonding cadmium
ions into the form of insoluble phosphates.
5. ACKNOWLEDGEMENTS
The work was supported by Grant No. N N508 38 2035 of the Min-
istry of Science and Higher Education of Poland.
REFERENCES
[1] Kabata-Pendias, A. and Pendias, H., (1993) Biochemistry
of trace elements. PWN Warsaw, Polish.
[2] Alloway, B.J. and Ayers, D.C. (1999) Chemical basis of
environmental pollution”. PWN Warsaw, Polish.
[3] Basta, N.T. Gradwohl, R. Snethen, K.L. and Schroder,
J.L. (2001) Chemical immobilization of lead, zinc and
cadmium in smaller-contaminated soils using biosolids
and rock phosphate. Journal of Environmental Quality,
30, 1222-1230. doi:10.2134/jeq2001.3041222x
[4] Raicevic, S. Kaludjerivic-Radoicic, T. and Zouboulis, A.I.
(2005) In situ stabilization of toxic metals in polluted
soils using phosphates: theoretical prediction and ex-
perimental verification. Journal of Hazardous Materials,
B117, 41-53. doi:10.1016/j.jhazmat.2004.07.024
[5] Gorlach, E. and Gambuś, F. (1997) Phosphorus and mul-
ticomponent fertilizers as a Skurce of soil pollution by
heavy metals. Zeszyty Problemowe Postępów Nauk Rol-
niczych, 448a, 139-146.
[6] Stoch, L., Stoch, Z. and Wacławska, I. Silicate glass fer-
tilizer. Patent PL, 185, 229 B1.
[7] Wacławska, I. and Szumera, M. (2009) Reactivity of sili-
cate-phosphate glasses in soil environment. Journal of
Alloys and Compounds, 468, 246-253.
doi:10.1016/j.jallcom.2007.12.093
[8] Lityński, T. Jurgowska and Gorlach, H.E. (1976) Che-
mical analysis for agriculture. PWN Warsaw, Polish.
[9] Hon, Y.M. Fung, K.Z. and Hon, M.H. (2001) Synthesis
and characterization of Li1+δMn2-δO4 powders prepared
by citric acid gel process. Journal of the European Ce-
ramic Society, 21, 515-522.
doi:10.1016/S0955-2219(00)00217-X
[10] Todorovsky, D.S. Todorovska, R.V. and Groudeva-Zoto-
va, St. (2002) Thermal decomposition of yttrium-iron cit-
rates prepared in ethylene glycol medium. Materials Let-
ters, 55, 41-45. doi:10.1016/S0167-577X(01)00616-4
[11] Maslowska, J. (1984) Thermal decomposition and ther-
mofractiochromatographic studies of metal citrates. Jour-