Paper Menu >>
Journal Menu >>
 Pharmacology & Pharmacy, 2011, 2, 42-46 doi:10.4236/pp.2011.21005 Published Online January 2011 (http://www.SciRP.org/journal/pp) Copyright © 2011 SciRes. PP Microwave Assisted Synthesis and Evaluation of Cross-Linked Carboxymethylated Sago Starch as Superdisintegrant Akhilesh V. Singh1, Lila K. Nath1, Manisha Guha2, Rakesh Kumar1 1Department of Pharmaceutical Sciences, Dibrugarh University-Dibrugarh, Assam, India; 2Grain Science and Technology Depart- ment, CFTRI, Mysore, Karnataka, India. Email: akhileshvikram@gmail.com Received October 20th, 2010; revised November 17th, 2010; accepted December 13th, 2010 ABSTRACT The aim of this study was to modify the sago starch and evaluate its efficacy as tablet disintegrant. Cross-linked car- boxymethylated sago starch (CMSS) was synthesized using native sago starch (SS) and monochloroacetic acid (MCA) with sodium hydroxide in microwave radiation environment. FT-IR analysis of the sample confirmed the carboxy- methylation by showing absorpt i on peak at 1607.2 cm -1. CMSS with degree of substitution (DS) of 0.31 was formed and, it was further evaluated as disintegrant in Ondasetron based tablets. The results revealed that CMSS could be used as disintegrant in tablet formulation in concentration dependant manner. Keywords: Sago Starch, Carboxymethylation, Disintegrant, SEM, FT-IR 1. Introduction Starch is one of the most important and abundant plant polysaccharide next to cellulose and chitin. Starch is found primarily in the seeds, fruits, tubers, and stem pith of plants, most notably corn, wheat, rice, sago, and pota- toes. Starch derivatives play vital roles in the promising biopolymers industries. This is because they are cheap, non-toxic, renewable and biocompatible with many other materials for industrial applications. Starches are em- ployed in food, pharmaceutical and allied industries be- cause of its good thickening and gelling properties. Starches can be modified by chemical, physical and en- zymatic methods for their tailor made use in different form [1]. They are mainly used as binder, filler, emulsion stabilizer, consistency modifier and disintegrantss. Crosslinked sodium carboxymethyl starch which is also known as sodium starch glycolate is extensively used in fast dissolving tablets to disperse the drugs within short span of time and deliver the active drug in the systemic circulation of the body. In a number of earlier reported methods, carboxy- methylation of starch has been done using strong NaOH and Mono chloroacetic acis (MCA) in aqueous/organic medium at elevated temperature. It has been shown that when a mixture of starch with sodium hydroxide and monochloroacetic acid is irradiated, the carboxymethyl starch obtained consists of fractions with high levels of carboxymethyl groups and fractions with a predominant content of carbonyl groups [2]. Earlier some workers have modified yam starch and evaluated its efficacy as tablet disintegrant [3]. In our laboratory recently we have synthesized carboxymethylated derivative of moth bean starch by conventional method and evaluated as su- perdisintegrant [4]. Microwave assisted synthesis is an efficient and novel technique used in polymer synthesis. Microwave-assisted synthesis has attracted huge interest in recent years among researchers due to its rapid transfer of energy in the bulk of the reaction, as well as short re- action time.Compared with the conventional approach a microwave-assisted reaction has advantages of energy saving, high conversion, and rapidity [5,6] . The sago palm (Metroxylon sagu Rottb.) is grown well in the tropical rain forests of Southeast Asia. This palm contains nearly 20-45% starch on dry weight basis in its trunk and it is one of the potential underutilized palms [7]. This study was designed to synthesize the crosslinked carboxymethyl derivative of sago starch, and evaluate its efficacy as disintegrant in pharmaceutical formulation. Ondasetron was chosen as model drug.  Microwave Assisted Synthesis and Evaluation of Cross-Linked Carboxymethylated Sago Starch as Superdisintegrant Copyright © 2011 SciRes. PP 43 2. Material and Methods 2.1. Materials Sodium starch glycolate and POCl3 was procured from Lobachemie, India. Ondasetron HCl was kindly donated by Comed Pharmaceuticals Limited, Baddi, India. Mono- chloro acetic acid (CDH, India) and Sodium hydroxide (Merck, India) were procured and used without further purification. Sago starch was procured from local market of Chennai, Tamilnadu, India. All other chemicals re- ceived were of AR grade and used without further puri- fication. 2.2. Methods 2.2.1. Modi fi cation of Sag o Sta rch Carboxymethylation of Sago starch (SS) was carried out by following slight modified method described elsewhere [8-10]. In this method Sago starch (4 g), NaOH (3.2 g) and monochloroacetic acid (4 g) were taken in a beaker, with 20 ml of Isopropyl alcohol and water (50:50) and the contents were subjected to continuous stirring to ho- mogeneity. Subsequent reaction was allowed to proceed at varying temperature (from 25˚C to 70˚C and duration of reaction (from 1 to 4 min) in the Microwave oven (Model No.CE1111TL, Samsung Electronics, India). The reaction products were precipitated with ethanol and washed alkali free and dried in a vacuum oven at 45˚C for 8 h. The reaction takes place in following manner: NaOH St-OH + CH2Cl-COOH St-O-CH2COOH + NaCl + H2O (1) Finally dried powder was further cross-linked with Phosphorous oxychloride (POCl3) to get the cross-linked Na-carboxymethylated sago starch (CMSS). 2.2.2. Degree of Substitution The DS of carboxymethylated sago starch (CMSS) was determined by the method reported elsewhere [11].The carboxymethyl groups in the CMSS were first converted to an acid form with acid (HCl). The acidified starch was then recovered by precipitation with methanol, filtration, washing with methanol, and drying. Then, 0.2 M NaOH (20 ml) was added to a suspension of accurately weighed CMSS in 30 ml of purified water. The mixture was transferred to a 100-ml volumetric flask and adjusted to the mark with purified water. The solution (25 ml) was transferred to an Erlenmeyer flask and titrated with 0.04 M HCl using phenolphthalein as the indicator. The titra- tion was repeated three times, and the average value of HCl volume was used for the calculations. A blank was also titrated. The DS was calculated using following equations: 162 nCOOH Degree of substitutionMDS-58 nCOOH (2) water 1W MDS MS 100 (3) bHCl nCOOHVV C 4 (4) where 162 is the molar mass of AGU (g/mol); nCOOH (mol) is the amount of COOH; MDS (g) is the mass of dry sample; MS (g) is sample mass: Wwater (%) is the water content; Vb (ml) is the volume of HCl used for the titration of the blank; V (ml) is the volume of HCl used for the titration of the sample; CHCl (mol/L) is the HCl concentration. 2.2.3. Scannin g Elec tr on Microscopy Each starch samples were firstly air dried, and then coated with gold. The prepared starch samples were viewed under scanning electron microscope (JEOL, Ja- pan). 2.2.4. FT-I R Study Both native and carboxymethylated starch sample (5 mg) were blended with solid KBr, (Merck, Germany) and about 40 mg blend was used to prepare a pellet (Hydrau- lic pellet press KP, Mumbai, India).The spectra were scanned from 4000-400 cm-1 in a Perkin Elmer FT-IR spectrometer(Perkin Elmer, USA) under dry air at room temperature. 2.2.5. Swelling Capacity The swelling capacity of the native SS, CMSS and SSG was estimated by slightly modified method of [12]. In this method the tapped volume occupied by 10 gm of the powder (Vx), was noted and the powder was dispersed in 85 ml of distilled water and the volume made up to 100 ml with distilled water. After 24 h of standing, the vol- ume of the sediment (Vv) was measured. The swelling capacity was then computed as the ratio of Vv/Vx. 2.2.6. Hydrati on C ap aci t y The slight modified method [13] was used for this study. A 10 gm was placed in each of four 15 ml plastic centri- fuge tubes to which 10 ml of distilled water was added and then stoppered. The contents were mixed on a cyc- lomixer for 2 min. The mixture was allowed to stand for 10 min and then centrifuged at 1000 rpm for 10 min on absented centrifuge. The supernatant was carefully decanted, and the sedi- ment was weighed. The hydration capacity (Hc) was then calculated using the equation: Sediment weight Hc =Dry sample weight (5) 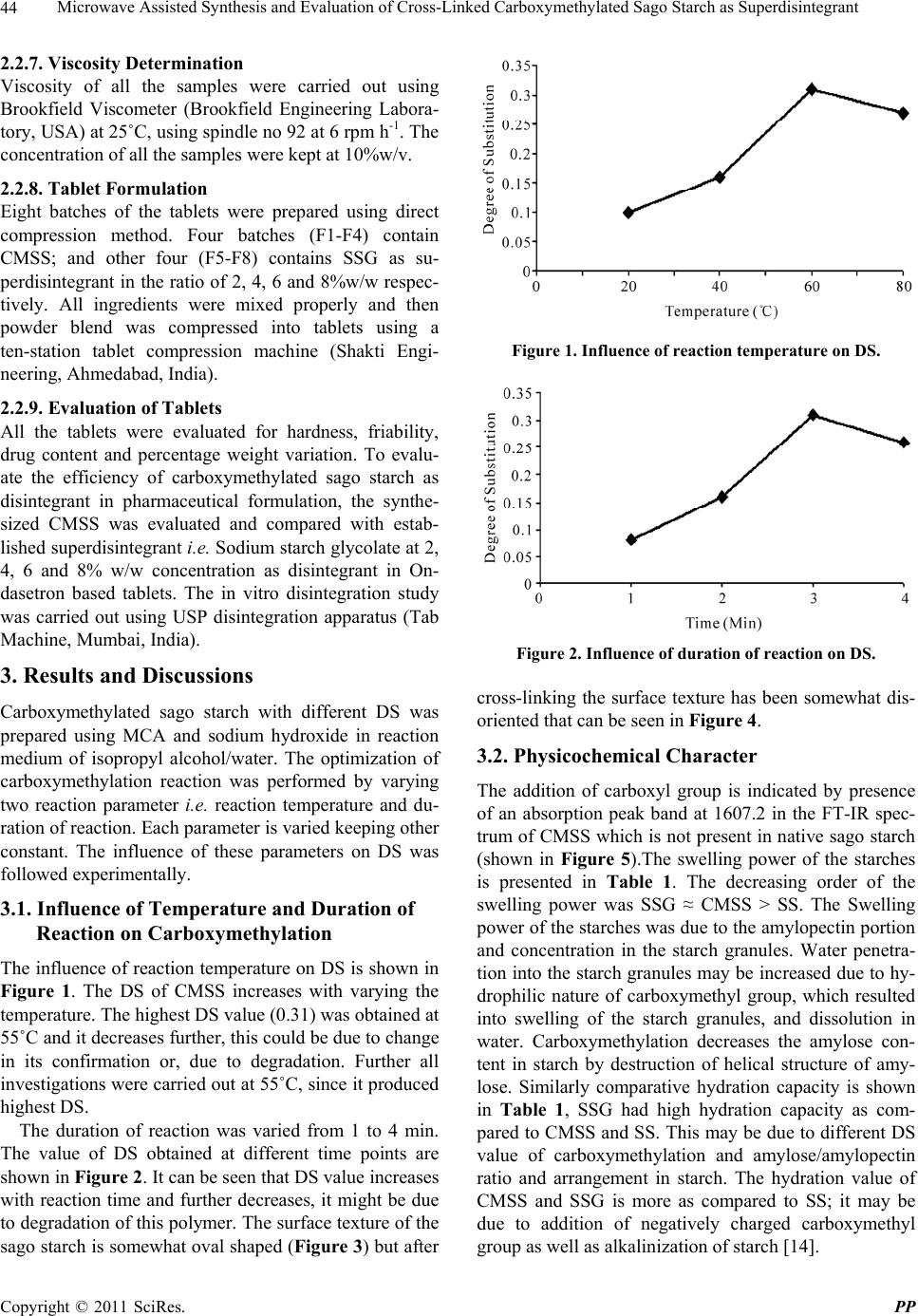 Microwave Assisted Synthesis and Evaluation of Cross-Linked Carboxymethylated Sago Starch as Superdisintegrant Copyright © 2011 SciRes. PP 44 2.2.7. Viscosity Determination Viscosity of all the samples were carried out using Brookfield Viscometer (Brookfield Engineering Labora- tory, USA) at 25˚C, using spindle no 92 at 6 rpm h-1. The concentration of all the samples were kept at 10%w/v. 2.2.8. Tablet Formulation Eight batches of the tablets were prepared using direct compression method. Four batches (F1-F4) contain CMSS; and other four (F5-F8) contains SSG as su- perdisintegrant in the ratio of 2, 4, 6 and 8%w/w respec- tively. All ingredients were mixed properly and then powder blend was compressed into tablets using a ten-station tablet compression machine (Shakti Engi- neering, Ahmedabad, India). 2.2.9. Evaluation of Tablets All the tablets were evaluated for hardness, friability, drug content and percentage weight variation. To evalu- ate the efficiency of carboxymethylated sago starch as disintegrant in pharmaceutical formulation, the synthe- sized CMSS was evaluated and compared with estab- lished superdisintegrant i.e. Sodium starch glycolate at 2, 4, 6 and 8% w/w concentration as disintegrant in On- dasetron based tablets. The in vitro disintegration study was carried out using USP disintegration apparatus (Tab Machine, Mumbai, India). 3. Results and Discussions Carboxymethylated sago starch with different DS was prepared using MCA and sodium hydroxide in reaction medium of isopropyl alcohol/water. The optimization of carboxymethylation reaction was performed by varying two reaction parameter i.e. reaction temperature and du- ration of reaction. Each parameter is varied keeping other constant. The influence of these parameters on DS was followed experimentally. 3.1. Influence of Temperature and Duration of Reaction on Carboxymethylation The influence of reaction temperature on DS is shown in Figure 1. The DS of CMSS increases with varying the temperature. The highest DS value (0.31) was obtained at 55˚C and it decreases further, this could be due to change in its confirmation or, due to degradation. Further all investigations were carried out at 55˚C, since it produced highest DS. The duration of reaction was varied from 1 to 4 min. The value of DS obtained at different time points are shown in Figure 2. It can be seen that DS value increases with reaction time and further decreases, it might be due to degradation of this polymer. The surface texture of the sago starch is somewhat oval shaped (Figure 3) but after Figure 1. Influence of reaction temperature on DS. Figure 2. Influence of duration of reaction on DS. cross-linking the surface texture has been somewhat dis- oriented that can be seen in Fi gu re 4. 3.2. Physicochemical Character The addition of carboxyl group is indicated by presence of an absorption peak band at 1607.2 in the FT-IR spec- trum of CMSS which is not present in native sago starch (shown in Figure 5).The swelling power of the starches is presented in Table 1. The decreasing order of the swelling power was SSG ≈ CMSS > SS. The Swelling power of the starches was due to the amylopectin portion and concentration in the starch granules. Water penetra- tion into the starch granules may be increased due to hy- drophilic nature of carboxymethyl group, which resulted into swelling of the starch granules, and dissolution in water. Carboxymethylation decreases the amylose con- tent in starch by destruction of helical structure of amy- lose. Similarly comparative hydration capacity is shown in Table 1, SSG had high hydration capacity as com- pared to CMSS and SS. This may be due to different DS value of carboxymethylation and amylose/amylopectin ratio and arrangement in starch. The hydration value of CMSS and SSG is more as compared to SS; it may be due to addition of negatively charged carboxymethyl group as well as alkalinization of starch [14].  Microwave Assisted Synthesis and Evaluation of Cross-Linked Carboxymethylated Sago Starch as Superdisintegrant Copyright © 2011 SciRes. PP 45 Figure 3. Scanning electron micrograph of native sago starch. Figure 4. Scanning electron micrograph of carboxymethy- lated sago starch. Figure 5. FT-IR spectra of native and modified sago starch. 3.3. Tablet Evaluation All the prepared tablets were evaluated for physical characterization and data shown in Table 2. The results were found within the official limits. Tablets containing different concentration of CMSS and SSG were evalu- ated for disintegrant property (shown in Figure 6). Car- boxymethylation of starch increases its cold-water hy- drophilicity due to addition of negatively charged func- tional group (CH2COO-) in the parent chain of native starch. The less concentration of CMSS possesses insuf- ficient swelling power to break the tablets. The tablets containing higher conc. (6% w/w) CMSS showed nearly comparable disintegration time as shown by SSG based tablets. At higher conc. (8% w/w) the disintegration time of both the tablets, mainly SSG based was decreased that may be due to formation of viscous gel mass which im- pede the penetration of water in to the tablets and re- tarded the disintegration power. The use of CMSS as a tablet disintegrant in this study seemed to have the opti- mum concentration at 8 %w/w. Table 1. Comparative swelling, hydration and viscosity behavior of all starches. Starch TypeSwelling capacity Hydration capacity Viscosity (cps) Sago Starch1.6 ± 0.10 0.68 ± 0.15 61343 CMSS 4.0 ± 0.21 2.2 ± 0.10 74012 SSG 4.2 ± 0.25 2.4 ± 0.25 75643 All the values are represented as Mean ± SD; and n = 3. Table 2. Physical Evaluation of tablets. Batch Hardness (kg/cm2) Friability (%) Drug content (%) % Weight deviation F1 4.6 ± 0.25 0.35 ± 0.26 97.35 ± 0.35 1.3 ± 0.18 F2 4.7 ± 0.37 0.33 ± 0.15 98.26 ± 0.20 1.0 ± 0.28 F3 4.3 ± 0.26 0.44 ± 0.21 99.13 ± 0.21 2.5 ± 0.20 F4 5.2 ± 0.31 0.53 ± 0.40 98.33 ± 0.26 2.7 ± 0.15 F5 4.9 ± 0.25 0.34 ± 0.35 99.43 ± 0.23 2.1 ± 0.35 F6 5.0 ± 0.22 0.22 ± 0.25 97.32 ± 0.20 2.4 ± 0.14 F7 5.2 ± 0.30 0.39 ± 0.16 98.48 ± 0.30 2.9 ± 0.20 F8 5.1 ± 0.10 0.36 ± 0.24 97.98 ± 0.34 2.1 ± 0.12 All the values are represented as Mean ± SD; and n = 3.  Microwave Assisted Synthesis and Evaluation of Cross-Linked Carboxymethylated Sago Starch as Superdisintegrant Copyright © 2011 SciRes. PP 46 Figure 6. Comparative disintegration behavior in ondase- tron based tablets. 4. Conclusion CMSS was synthesized in microwave radiation environ- ment with DS value of 0.31 using MCA and NaOH in IPA/H2O solvent medium. The physicochemical values are comparable to official superdisintegrant i.e. SSG, but much higher than native sago starch. CMSS could be used as a potential disintegrant in tablet dosage form at higher concentration (8% w/w). 5. Acknowledgement The authors are thankful to Prof. Harkesh B Singh, De- partment of Chemistry, IIT-Bomaby for carrying out FT-IR of the samples, and also to IIT-Guwahati for helping in SEM characterization. REFERENCES [1] O. B. Wurzburg, “Modified Starches: Properties and Uses,” CRC-Press, Florida, 1984, pp. 4-15. [2] O. S. Kittipongpatana, J. Sirithunyalug and R. Laenger, “Preparation and Physicochemical Properties of Sodium Carboxymethyl Mungbean Starches,” Carbohydrate Poly- mer, Vol. 63, No. 1, 2006, pp. 105-112. doi:10.1016/j.carbpol.2005.08.024 [3] N. Nattapulwat, N. Purkkao and O. Suwihaayapan, “Preparation and Application of Carboxymethyl Yam (Dioscorea Esculenta) Starch,” AAPS PharmSciTechnol- ogy, Vol. 10, No. 1, 2009, pp. 193-198. doi:10.1208/s12249-009-9194-5 [4] A. V. Singh, L. K. Nath, R. D. Pandey and A. Das, “Syn- thesis and Application of Moth Bean Starch as Superdis- integrant in Pharmaceutical Formulation,” Journal of Polymer Materials, Vol. 27, No. 2, 2010, pp. 173-177. [5] V. Singh and A. Tiwari, “Microwave Accelerated Me- thylation of Starch,” Carbohydrate Research, Vol. 343, No. 1, 2008, pp. 151-154. doi:10.1016/j.carres.2007.09.006 [6] A. N. Jyothia, K. N. Rajasekharanb, S. N. Moorthy and J, Sreekumara, “Microwave Assisted Synthesis and Char- acterization of Succinate Derivative of Cassava (Manihot Esculenta) Starch,” Starch/Starke, Vol. 57, No. 11, 2005, pp. 556-563. doi:10.1002/star.200500429 [7] Z. A. Noor Fadzlina, A. A. Karim and T. T. Teng, “Phys- icochemical Property of Carboxymethyl Sago ( Metroxy- lan Sagu) Starch,” Journal of Food Science, Vol. 70, No. 9, 2005, pp. 560-567. doi:10.1111/j.1365-2621.2005.tb08305.x [8] S. Zeiko, J. J. Katarina and J. Slobodan, “Synthesis of Carboxymethyl Starch,” Starch/Starke, Vol. 52, No. 11, 2000, pp. 413-419. doi:10.1002/1521-379X(200011)52:11<413::AID-STAR 413>3.0.CO;2-B [9] P. D. Pandya, N. K. Patel and V. K. Sinha, “Synthesis and Characterization of Sodium Salt of Partially Carboxy- methylated Starch,” International Journal of Polymeric Materials, Vol. 51, No. 12, 2002, pp. 1081-1085. doi:10.1080/714975698 [10] S. Kamel and K. Jahangir, “Optimization of Carboxy- methylation of Starch in Organic Solvents,” International Journal of Polymeric Materials, Vol. 56, No. 5, 2007, pp. 511-519. doi:10.1080/00914030600945770 [11] Z. Stojanovic, K. Jeramics, S. Jovanovic and D. M. Lechner, “A Comparison of Some methods for the De- termination of the Degree of Substitution of Carboxy- methyl starch,” Starch/Starke, Vol. 51, No. 1, 2005, pp. 79-83. doi:10.1002/star.200400342 [12] A. O. Okhamafe, A. Igboechi and T. O. Obasaki, “Cellu- loses Extracted from Groundnut Shell and Rice Husk. 1: Preliminary Physicochemical Characterization,” Phar- macy World Journal, Vol. 8, No. 4, 1991, pp. 121-123. [13] S. S. Komblum and S. B. Stoopak, “A New Tablet Disin- tegrant: Crosslinked Polyvinylpyrrolidine,” Journal of Pharmaceutical Sciences, Vol. 62, No. 1, 1973, pp. 43-49. doi:10.1002/ jps.2600620107 [14] M. I. Khalil, A. Hashem and A. Hebesish, “A Carboxy- methylation of Maize Starch,” Starch/Starke, Vol. 42, No. 2, 1990, pp. 60-63. doi:10.1002/star.19900420209 |

