Food and Nutrition Sciences
Vol. 4 No. 8A (2013) , Article ID: 35325 , 5 pages DOI:10.4236/fns.2013.48A028
Influence of Micronized Chitosan on Antioxidative Activities in Grape Juice
![]()
1Department of Horticulture and Biotechnology, Chinese Culture University, Taipei, Chinese Taipei; 2Graduate Institute of Biotechnology, Chinese Culture University, Taipei, Chinese Taipei.
Email: *pojungchien@gmail.com
Copyright © 2013 Po-Jung Chien et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received June 5th, 2013; revised July 5th, 2013; accepted July 12th, 2013
Keywords: Micronization; Chitosan; Grape Juice; Antioxidative Activity
ABSTRACT
The antioxidant activity of chitosan before micronization (BMC, average particle size of 1850 ± 26.3 μm) and after micronization (AMC, average particle size of 1.37 ± 0.2 μm) in grape juice was studied. Antioxidant activity was determined, including that of DPPH radicals, hydrogen peroxide and superoxide anion radicals, as well as ABTS radicals of BMC or AMC in grape juice. AMC exhibits stronger scavenging activity toward DPPH radicals, superoxide anion radicals and hydrogen peroxide than BMC. At a concentration of 1.0 mg/mL, AMC in grape juice exhibited 90.0%, 97.3% and 88.7% scavenging activities toward DPPH radicals, hydrogen peroxide and superoxide anion radicals, respectively. The TEAC (Trolox Equivalent Antioxidant Capacity) values of AMC (3.94 ± 0.19) greatly exceeded those of BMC (2.21 ± 0.10) in grape juice. The in vitro results in this investigation suggest the possibility that AMC can increase the antioxidant activity in grape juice. However, comprehensive studies must be performed to ascertain the in vivo safety of AMC in experimental animal models.
1. Introduction
Chitosan (β-(1-4)-2-amino-2-deoxy-D-glucose) is a linear hydrophilic polysaccharide polymer of d-glucosamine. It is abundant in nature and is present in the exoskeleton of crustaceans, including crabs and shrimp [1]. Chitosan, being a cationic polysaccharide under neutral or basic pH conditions, contains free amino groups and is therefore insoluble in water. At acidic pH values, amino groups undergo protonation thus, making chitosan soluble in water. It breaks down slowly into harmless products (amino sugars), which are entirely absorbed by the human body [2].
In recent years, various researchers have evaluated the antioxidant activity of chitosan derivatives. For example, Xie et al. [3] revealed that hexanolychitin and N-benzoylhexanoyl chitosan can trap peroxide radicals in an organic solvent when 2,2’-azobis (2,4-dimethylvaleronitrile) initiates the radical chain reaction. Lin and Chou [4] demonstrated that disaccharide chitosan derivatives exhibit a range of antioxidative activities. Esumi et al. [5] showed that gold-chitosan nanocomposites can suppress the activity of hydroxyl radicals. Xing et al. [6] determined the effects of molecular weight and/or the degree of substitution of sulfated polysaccharides on their antioxidant activity. The health advantages of consuming grape juice, including improved endothelial function, increased antioxidant capacity of serum, protection of LDLs against oxidation, reduced native plasma protein oxidation, and reduced platelet aggregation, have been reported upon [7]. However, the effect of micronized chitosan on the antioxidant activity of grape juice has not yet been reported upon. This study compares the effects of micronized chitosan on the antioxidant activity, and on the possible antioxidant effects of grape juice. This investigation will evaluate the antioxidant capacity of BMC (or AMC) in grape juice. Antioxidant activities were evaluated using various in vitro assay systems, involving, for example, DPPH, superoxide, hydroxyl radicals and ABTS.
2. Materials and Methods
2.1. Micronization of Chitosan and Chemicals
Crab-shell chitosan with 86.70% N-deacetylation as a powder were obtained from VA & G Bioscience Inc (Taoyuan, Taiwan) and micronzied using a high-speed planetary ball-mill (PM100, Retsch, Germany), by the approach of that was proposed by Chau et al. [8]. In the ball-milling process, sample and agate balls with a diameter of 3 mm in a volume ratio of 1:1 (~165 ml each) were added to an agate grinding bowl with a volume of 500 ml. The sample was then ground for 2 h using agate balls. Before and after the chitosan samples were estimated using a laser particle size analyzer (Analysette 22-Economy, Fritsch, Germany). All other chemicals were obtained commercially and were of analytical grade. Nitro blue tetrazolium (NBT), phenazine methosulfate (PMS), hydrogen peroxide (H2O2), thiobarbituric acid (TBA), ethylene diamine tetra-acetic acid (EDTA), ferrozine, nicotinamide adenine dinucleotide-reduced (NADH), trichloroacetic acid (TCA), potassium ferricyanide and ferric chloride were obtained from Sigma Chemicals Co., St. Louis, MO, USA.
The antioxidant activities of micronized individual chitosans were evaluated in grape juice.
2.2. Preparing Chitosan Solution
In the preparation of 1.0 L of 0.1% - 1.0% chitosan solutions, 0.1, 0.5 and 1.0 g of chitosan were dispersed in 900 ml of distilled water, to which 50 ml of glacial acetic acid was added to dissolve the chitosan. The pH of each solution was adjusted to 5.0 by adding 0.1 M NaOH and each solution was made up to 1.0 L. An acid solution without chitosan at pH 5.0 was used as a control.
2.3. Preparing Grape Juice That Contains Micronized Chitosans
Clear, UTH-treated, shelf-stable grape juice that contained no added preservatives and was packed in laminated, was purchased from a local retailer. To 45 mL of this grape juice in a 250 mL Erlenmeyer flask was added 5 mL of micronized chitosan solution. To the control flask was added 5 mL of water, rather than chitosan solution. The used chitosan concentrations ranged from 0.1 to 1.0 g/L of juice.
2.4. Scavenging of DPPH Radical
The effect of chitosans on DPPH radicals was examined using the modified method of Shimada et al. [9]. Briefly, 100 μM DPPH solution in methanol was prepared and 1.0 mL of this solution was added to 4.0 mL test samples at (various concentrations. The reaction mixture was shaken thoroughly and incubated for 30 min at room temperature and the absorbance of the resulting solution was determined at 517 nm against a blank. The percentage inhibition of DPPH was calculated using the following equation:
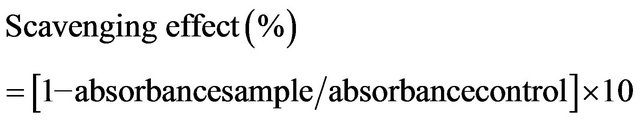
2.5. Hydrogen Peroxide Scavenging Assay
The activity of chitosan in scavenging hydrogen peroxide was measured by a modified version of the method that was proposed by Yen and Chang [10]. Briefly, 1 mL of sample was firstly mixed with 400 μL of 4 mM H2O2 solution and incubated for 20 min at room temperature. It was then supplemented with 600 μl of horseradish peroxidase—phenol red solution (HPRase 300 μg/mL and phenol red 4.5 mM in 100 mM phosphate buffer). HPRase was produced by and obtained from Sigma Chemical Co, St Louis, MO, USA. After another 10 min of incubation and 10 min on ice to terminate the reaction, the absorbance of the sample at 610 nm was monitored using an automated microplate reader. The scavenging effect was then calculated using the equation,
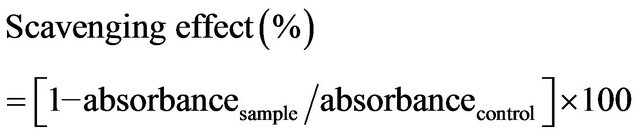
2.6. Superoxide Anion Radical Scavenging Assay
The superoxide scavenging capacity of chitosans was assayed using the method of Robak and Gryglewski [11]. The reaction mixture contained chitosans at concentration of 0.1 - 10 mg/mL. First, the reagents were all prepared in 100 mM phosphate buffer (pH 7.4). The reaction mixture contained 50 μL of the test sample, 50 μL of 300 μM nitrobluetetrazolium (Sigma), 50 μL of 936 μM NADH and 50 μL of 120 μM phenazine methosulfate (Sigma). It was incubated at room temperature for 5 min and the absorbance was then read at 560 nm against a blank. The capacity of chitosan to scavenge superoxide radicals was given by the following equation.
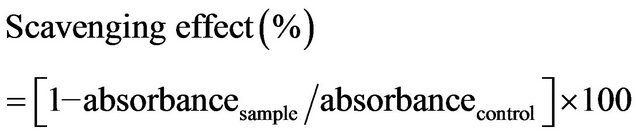
2.7. ABTS Assay
Total antioxidant capacity was evaluated using the ABTS modified assay [12-14]. In the most recent version of the trolox equivalent antioxidant capacity (TEAC) assay, an antioxidant is added to a pre-formed ABTS radical solution and, after a fixed period, the remaining ABTS●+ is quantified spectrophotometrically [15]. The reference compound in the TEAC assay is Trolox. The reduction in ABTS●+ concentration, which is caused by a particular concentration of antioxidant, is related to reduction of Trolox concentration, and yields the TEAC value of that antioxidant [13].
ABTS●+ was formed by reacting 2.45 mM ABTS salt, of potassium persulfate (K2S2O8), was reacted with 7 mM ABTS salt in 0.01 M phosphate-buffered saline, pH7.4, for 15 h at room temperature in the dark. The resulting ABTS●+ radical cations were diluted with 0.01 M phosphate-buffered saline at pH 7.4 to yield an absorbance of approximately 0.70 at 734 nm. The standard or sample was diluted by a factor of 100 using the ABTS●+ solution to a total volume of 1mL and allowed to react for 6 min. Absorbance was measured spectrophotometrically at various times. A blank (without a standard or sample) was used as a control and 990 μL of PBS was added to the control samples instead. The absorption peak of ABTS●+ was at 734 nm. The addition of antioxidant reduced ABTS●+ to its colorless form. The extent of decolorization as a percentage of inhibition of ABTS●+ is determined as a function of concentration and calculated relative to the reactivity of Trolox, a water-soluble analog of vitamin E (α-tocopherol).
2.8. Statistical Analysis
In this investigation, three analyses of each sample were performed and each experiment was conducted in triplicate (n = 3). The mean value and its standard deviation were calculated from the obtained data. These data were then compared with each other using Duncan’s multiple range method.
3. Results and Discussion
3.1. Scavenging of DPPH Radicals by BMC or AMC
The DPPH, a compound with a proton free radical and a characteristic absorption at 517 nm, is decreased greatly upon exposure to proton radical scavengers [16]. In this study, DPPH was utilized to determine the proton scavenging activity of chitosans of BMC or AMC.
Figure 1 plots the DPPH scavenging potentials of BMC and AMC in grape juice. In the DPPH test, the grape juice reduced the DPPH radicals, exhibiting 21% scavenging activity. Here, AMC in grape juice exhibited excellent scavenging activity toward DPPH radical. This fact is attributable to the fact that it has a greater hydrogen-donating capacity than does grape juice (control), BMC or AMC. At a concentration of 1.0 mg/mL, the AMC in grape juice scavenged 90% of DPPH radicals. The data herein concerning the DPPH scavenging potential of BMC in grape juice demonstrate that AMC probably contributed greatly to the observed antioxidant effect.
3.2. Scavenging Activity of BMC or AMC toward Superoxide Anion Radical
Superoxides are radicals whose unpaired electrons are on oxygen. While they are relatively weak oxidants, superoxides exhibit limited chemical reactivity, but can form more dangerous species, including singlet oxygen and hydroxyl radicals, which cause the peroxidation of lipids [17].
Figure 2 shows the effect of BMC or AMC on the superoxide anion radical scavenging activities of grape juice. The data show that AMC had stronger scavenging activity toward superoxide anion radicals at concentrations of 1.0 mg/mL than at 0.1 mg/mL (p < 0.05). However, AMC had an excellent capacity to scavenge superoxide anion radicals in grape juice. It had the highest scavenging activity in the elimination of superoxide anion radicals (p < 0.05) of any of the tested compounds. At a concentration of 1.0 mg/mL, the AMC in grape
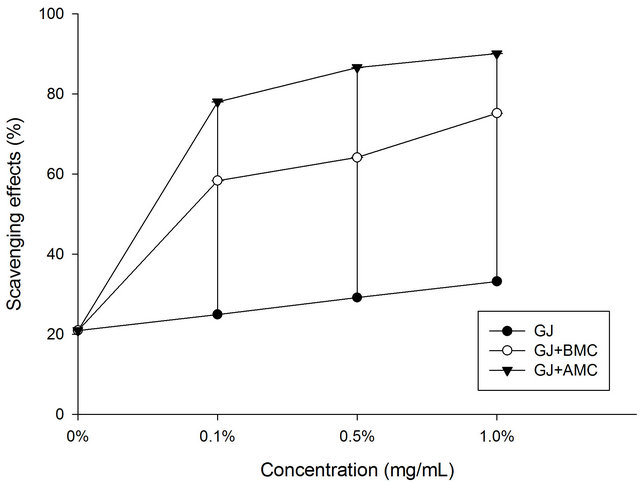
Figure 1. Scavenging effects of BMC or AMC (chitosans) in grape juice on DPPH radicals. Each value is presented as mean ± SD (n = 3). Grape juice, ˜; Grape juice + BMC, ™; Grape juice + AMC, q.
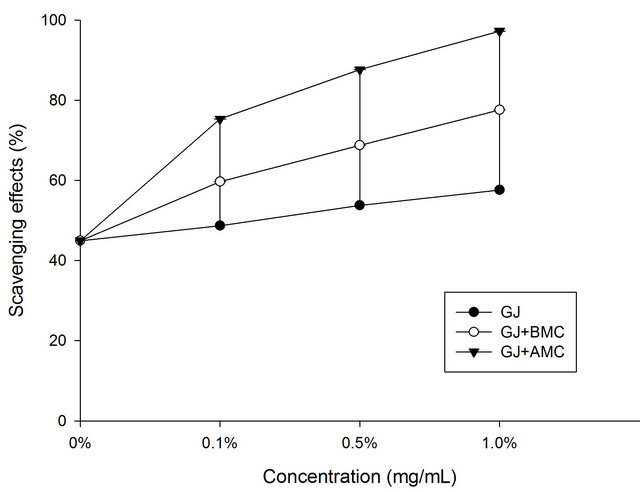
Figure 2. Scavenging effects of BMC or AMC in grape juice on hydrogen peroxide. Each value is presented as mean ± SD (n = 3). Grape juice, ˜; Grape juice + BMC, ™; Grape juice + AMC, q.
juice scavenged 97.3% of superoxide anion radicals. They may be more soluble than BMCs. Moreover, superoxides are also known to initiate indirectly lipid peroxidation as a result of the formation of H2O2, forming precursors to hydroxyl radicals [18]. These findings clearly reveal that the antioxidant activity of AMC increased the capacity of grape juice to scavenge superoxide anion radicals.
3.3. Hydrogen Peroxide-Scavenging Activity of BMC or AMC
Some biological systems can generate hydrogen peroxide. High concentrations of hydrogen peroxide can attack various cellular energy-producing systems; as an example of its detrimental effects, it deactivates the glycolytic enzyme glyceraldehydes-3-phosphate dehydrogenase [18]. As a non-radical oxygen-containing reactive agent, it can form the hydroxyl radical, which is the most highly reactive known oxygen radical, in the presence of transition metal ions and thereby participate in a free-radical reaction [19].
Figure 3 shows the hydrogen peroxide scavenging activities of BMC or AMC in grape juice. In the hydrogen peroxide test, the grape juice reduced hydrogen peroxide with a scavenging activity of 25%. In this work, AMC increases the H2O2-scavenging activity of grape juice. Moreover, the scavenging rate of the chitosans other than BMC increased with concentration. At a concentration of 1.0 mg/mL, the AMC in grape juice scavenged 88.7% of hydrogen peroxide, revealing that the tested BMC and AMC also exhibit considerable H2O2-scavenging activity in grape juice. AMC was more effective in scavenging H2O2 in grape juice than was grape juice alone (control) or BMC in grape juice (p < 0.05). The chealting effects
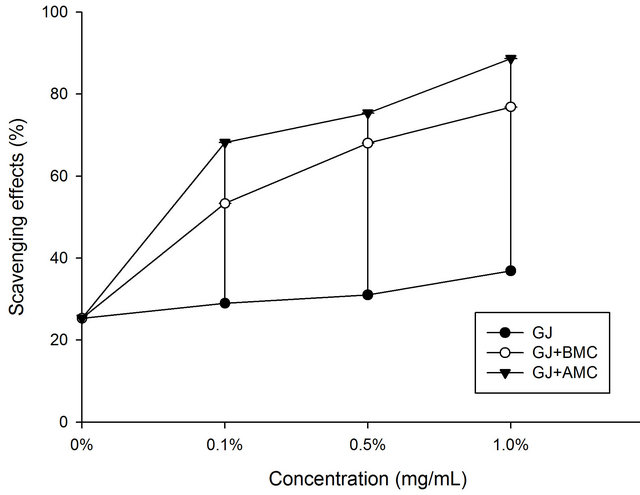
Figure 3. Scavenging effects of BMC or AMC in grape juice on superoxide anion radicals. Each value is presented as mean ± SD (n = 3). Grape juice, ˜; Grape juice + BMC, ™; Grape juice + AMC, q.
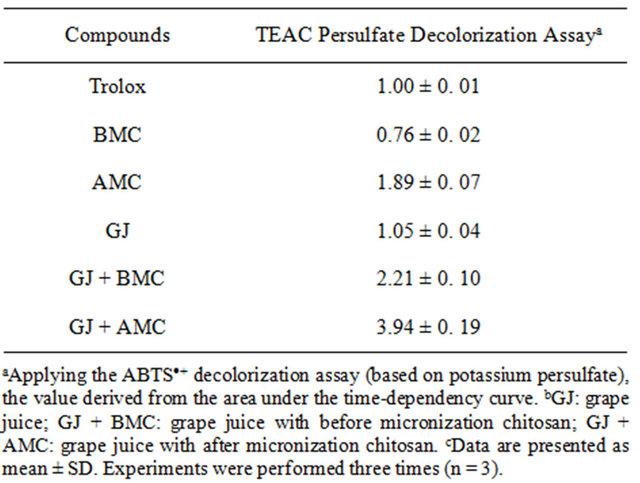
Table 1. The Trolox equivalent antioxidant capacity (TEAC) value of BMC, AMC in grape juices.
of AMC on metal ions may be responsible for its inhibittion of deoxyribose oxidation.
3.4. Effect of Trolox Equivalent Antioxidant Capacity (TEAC) on BMC or AMC
Table 1 presents the effect of BMC or AMC on antioxidant capacity, expressed as TEAC. The behaviors of BMC and AMC alone differ from those in grape juice. BMC reduced the TEAC value of grape juice to 0.76 ± 0.02. However, AMC increased the TEAC value of grape juice to 3.94 ± 0.19. The TEAC values of the flavanones, obtained from radical DPPH, were lower than those obtained from the radical ABTS [20]. A possible cause of the considerable rise in TEAC of grape juice is the increase in antioxidant potential polyphenols in the intermediate stages of oxidation. However, further investigations must be performed to elucidate the antioxidant mechanisms of AMC in grape juice.
4. Conclusion
The results of this work indicate that AMC (chitosan) exhibit antioxidant activity and free radical scavenging activity, including toward DPPH radicals, hydrogen peroxide and superoxide anion radicals. The assays herein were useful in determining the antioxidant capacities of AMC, which have important applications in the food industry. This study showed that AMC can increase the antioxidant activity of grape juice. However, in vivo antioxidant activity and its various antioxidant mechanisms must be examined further.
5. Acknowledgements
The authors would like to thank the National Science Council of the Republic of China, Taiwan (Contract No. NSC96-2313-B-034-006-MY3) and VA&G Bioscience Inc. for financially supporting this research.
REFERENCES
- M. Fukuda, N. A. Peppas and J. W. McGinity, “Properties of Sustained Release Hot-Melt Extruded Tablets Containing Chitosan and Xanthan Gum,” International Journal of Pharmaceutics, Vol. 310, No. 1-2, 2006, pp. 90- 100. doi:10.1016/j.ijpharm.2005.11.042
- S. A. Agnihotri, N. N. Mallikarjuna and T. M. Aminabhavi, “Recent Advances on Chitosan-Based Microand Nanoparticles in Drug Delivery,” Journal of Controlled Release, Vol. 100, No. 1, 2004, pp. 5-28. doi:10.1016/j.jconrel.2004.08.010
- W. Xie, P. Xu and Q. Liu, “Antioxidative Activity of Water Soluble Chitosan Derivatives,” Bioorganic and Medicinal Chemistry Letters, Vol. 11, No. 1, 2001, pp. 1699- 1701. doi:10.1016/S0960-894X(01)00285-2
- H. Y. Lin and C. C. Chou, “Antioxidative Activities of Water-Soluble Disaccharide Chitosan Derivatives,” Food Research International, Vol. 37, No. 9, 2004, pp. 883-889. doi:10.1016/j.foodres.2004.04.007
- K. Esumi, N. Takei and T. Yoshimura, “Antioxidant-Potentiality of Gold-Chitosan Nanocomposites,” Colloids and Surfaces B: Biointerfaces, Vol. 32, No. 2, 2003, pp. 117- 123. doi:10.1016/S0927-7765(03)00151-6
- R. Xing, S. Liu, H. Yu, Q. Zhang, Z. Li and P. Li, “Preparation of Low-Molecular-Weight and High-Sulfate-Content Chitosans under Microwave Radiation and Their Potential Antioxidant Activity in Vitro,” Carbohydrate Research, Vol. 339, No. 15, 2004, pp. 2515-2519. doi:10.1016/j.carres.2004.08.013
- A. Dávalos, B. Bartolomé and C. Gómez-Cordovés, “Antioxidant Properties of Commercial Grape Juices and Vinegars,” Food Chemistry, Vol. 93, No. 2, 2005, pp. 325- 330. doi:10.1016/j.foodchem.2004.09.030
- C. F. Chau, Y. L. Wen and Y. T. Wang, “Effects of the Micronization on the Characteristics and Physicochemical Properties of Insoluble Fibres,” Journal of the Science of Food and Agriculture, Vol. 86, No. 14, 2006, pp. 2380- 2386. doi:10.1002/jsfa.2628
- K. Shimada, K. Fujikawa, K. Yahara and T. Nakamura, “Antioxidative Properties of Xanthan on the Anti-Oxidation of Soybean Oil in Cyclodextrin Emulsion,” Journal of Agricultural and Food Chemistry, Vol. 40, No. 6, 1992, pp. 945-948. doi:10.1021/jf00018a005
- G. C. Yen and D. Y. Chung, “Antioxidant Effects of Extracts from Cassia tora L. Prepared under Different Degrees of Roasting on the Oxidative Damage to Biomolecules,” Journal of Food Chemistry, Vol. 47, No. 4, 1999, pp. 1326-1332. doi:10.1021/jf9810618
- J. Robak and R. J. Gryglewski, “Flavonoids Are Scavengers of Superoxide Anions,” Biochemical Pharmacology, Vol. 37, No. 5, 1988, pp. 837-841. doi:10.1016/0006-2952(88)90169-4
- R. Pellegrini, N. Proteggente, A. Pannala, A. Yang and C. Rice-Evans, “Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay,” Free Radical Biology & Medicine, Vol. 26, No. 9-10, 1999, pp. 1231- 1237. doi:10.1016/S0891-5849(98)00315-3
- M. J. T. J. Arts, J. S. Dallinga, H.-P. Voss, G. R. M. M. Haenen and A. Bast, “A New Approach to Assess the Total Antioxidant Capacity Using the TEAC Assay,” Food Chemistry, Vol. 88, No. 4, 2004, pp. 567-570. doi:10.1016/j.foodchem.2004.02.008
- L. C. Wu, H. W. Hsu, Y. C. Chen, C. C. Chiu, Y. I. Lin and J. A. Ho, “Antioxidant and Antiproliferative Activities of Red Pitaya,” Food Chemistry, Vol. 95, No. 2, 2006, pp. 319-327. doi:10.1016/j.foodchem.2005.01.002
- R. Berg van den, G. R. M. M. Haenen, H. Berg van den and A. Bast, “Applicability of an Improved Trolox Equivalent Antioxidant Capacity (TEAC) Assay for Evaluation of Antioxidant Capacity Measurements of Mixtures,” Food Chemistry, Vol. 66, No. 4, 1999, pp. 511-517. doi:10.1016/S0308-8146(99)00089-8
- T. Yamaguchi, H. Takamura, T. Matoba and J. Terao, “HPLC Method for Evaluation of the Free Radical-Scavenging Activity of Foods by Using 1,1-Diphenyl-2-picrylhydrazyl. Bioscience,” Biotechnology and Biochemistry, Vol. 62, No. 6, 1998, pp. 1201-1204. doi:10.1271/bbb.62.1201
- B. Halliwell and S. Chirico, “Lipid Peroxidation: Its Mechanism, Measurement, and Significance,” The American Journal of Clinical Nutrition, Vol. 57, No. 5, 1993, pp. 715-725.
- P. A. Hyslop, D. B. Hinshaw, W. A. J. Halsey, I. U. Schraufstatter, R. D. Sauerheber, R. G. Spragg, J. H. Jackson and C. G. Cochrane, “Mechanisms of Oxidant-Mediated Cell Injury the Glycolytic and Mitochondrial Pathways of ADP Phosphorylation Are Major Intracellular Targets Inactivated by Hydrogen Peroxide,” The Journal of Biological Chemistry, Vol. 263, No. 4, 1988, pp. 1665-1675.
- B. Halliwell, M. A. Murcia, S. Chirico and O. I. Aruoma, “Aruoma, Free Radicals and Antioxidants in Food and in Vivo: What They Do and How They Work,” Critical Reviews in Food Science and Nutrition, Vol. 35, No. 1-2, 1995, pp. 7-20. doi:10.1080/10408399509527682
- P. T. Gardner, T. A. C. White, D. B. McPhail and G. G. Duthie, “The Relative Contribution of Vitamin C, Carotenoids and Phenolics to the Antioxidant Potential of Fruit Juices,” Food Chemistry, Vol. 68, No. 4, 2000, pp. 471- 474. doi:10.1016/S0308-8146(99)00225-3
NOTES
*Corresponding author.

