 Vol.2, No.3, 272-278 (2010) doi:10.4236/health.2010.23039 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ Health Higher expression of connexin 40 in human atrial tissue of patients with type 2 diabetes who have undergone a coronary artery bypass graft surgery Pascal Daleau1,2, Geneviève Comeau1, Dominique Fournier1, Dany Patoine1, Patrick Mathieu1,3, Paul Poirier1,2 1Quebec Heart and Lungs Institute, Laval Hospital Research Centre, Quebec, Canada; pascal.daleau@pha.ulaval.ca 2Faculty of Pharmacy, Laval University, Quebec, Canada 3Faculty of Medicine, Laval University, Quebec, Canada Received 21 November 2009; revised 13 January 2010; accepted 16 January 2010. ABSTRACT Background: Although cardiac-related mortality rates are declining for the general population in the United States, this is not the case for pa- tients with diabetes. Diabetes is a significant independent predictor of atrial fibrillation (AF), the most common cardiac rhythm disturbance responsible for substantial morbidity and mortality. Objectives: This research was designed to evaluate properties of the atrial tissue between patients with and without type 2 diabetes. Heart rate variability (HRV) indices were calculated and expression of Kv1.5, connexin 43 (Cx43), and 40 (Cx40) were compared. Methods: Pa- tients undergoing a CABG were enrolled: 10 with type 2 diabetes and 8 without diabetes, paired for age, gender and co-morbidities such as hypertension and dyslipidemia. All patients showed normal ejection fraction. A sample of right auricular appendix was taken during CABG and Kv1.5, Cx40 and Cx43 protein contents were determined by western blotting and normalized to α-tubulin level. Results: No HRV difference was found between patients with and without diabetes. Cx43 and Kv1.5 levels were unaffected by diabetes (p=0.20 and 0.07, respectively) whe- reas Cx40 content was significantly increased by 55% (p=0.02). Levels of Cx43 phosphorylated and non-phosphorylated forms were non-sig- nificantly decreased in patients with diabetes. Conclusion: Patients with type 2 diabetes had higher expression of Cx40 in the right auricular appendix tissue. In light of other studies having demonstrated a link between AF and Cx40 ex- pression, it is possible that higher prevalence of AF in patients with diabetes is explained, at least partially, by differential expression of gap-junc- tion proteins. Keywords: Diabetes; Gap Junctions; Atrial Tissue; Atrial Fibrillation 1. INTRODUCTION The prevalence of diabetes is steadily increasing in west- ernized societies as well as in developing countries. Al- though the overall cardiac-related mortality rate has been declining for the general population in the United States, this is not the case for patients with diabetes [1]. Of par- ticular significance, diabetes is a risk factor for different heart-related conditions such as coronary artery disease, heart failure, aortic stenosis and arrhythmias [2]. Atrial fibrillation (AF) is the most common arrhyth- mia and is responsible for substantial morbidity and mortality [3]. In the Framingham study, systemic hyper- tension and diabetes were found to be independent pre- dictors of AF [4]. For men and women, respectively, diabetes increased by 1.4 and 1.6 fold the risk of devel- oping AF. The risk of developing AF correlates with atrial dilatation, and the presence of left ventricular dia- stolic dysfunction [5]. Since AF occurs as an ongoing process over time, subtle atrial abnormalities may pre- cede overt atrial dilatation and contractile dysfunction [5-7]. These could be linked to alterations in electro- physiological properties predisposing to AF. In animal models (mostly streptozotocin-induced dia- betes models), diabetes mellitus is responsible for car- diac arrhythmias, probably caused by an excessive lengthening of the cardiac action potential [8]. Several changes in ionic currents have been described in car- diomyocytes isolated from animal models of diabetes, principally a decrease in the transient repolarizing potas- sium current Ito [8,9]. This effect could possibly be par- tially mediated by impaired sympathetic nervous system  P. Daleau et al. / HEALTH 2 (2010) 271-277 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 273 activity [10] or by oxidative stress-induced alteration in the glutathione redox state [11]. It should be pointed out that a reduction in Vmax, [12] the maximum speed of the rising phase of the action potential, and a prolongation of the QRS complex [13, 14] have been associated with diabetes. Changes in the intercellular electrical coupling through gap junctions could be involved in this effect. Indeed, it was demon- strated that elevated glucose concentrations inhibit gap junction intercellular communication and reduced con- nexin (Cx) expression in a variety of cells including vascular smooth muscle cells, endothelial cells, and retinal microvessels. It has been proposed that high glu- cose concentration interfered with gap junction through the activation of protein kinase C [15-18]. In addition, it has been reported in rat model of diabetes that a down-regulation of Cx43 occurred and is associated with a compensatory over-expression of other Cxs [19,20]. However, direct alteration of gap junctions by diabetes were never assessed in human cardiomyocytes. The purpose of this study was to evaluate properties of the atrial tissue between patients with and without type 2 diabetes. Heart rate variability (HRV) indices were calculated to evaluate the potential influence of the cardiac autonomic nervous system. Expression levels of Kv1.5 (underlying IKur), connexin 43 (Cx43), and 40 (Cx40) were compared between patients with and with- out type 2 diabetes. 2. RESEARCH DESIGN AND METHODS 2.1. Study Design Ten subjects with type 2 diabetes and 8 subjects without diabetes undergoing a coronary artery bypass grafting surgery (CABG) were enrolled in this study. Patients in sinus rhythm aged between 35 and 70 were included. Patients with and without type 2 diabetes were paired for age, gender, co-morbidities such as hypertension and dyslipidemia. All patients had to show normal ejection fraction. Exclusion criteria were: 1) previous history of supraventricular arrhythmia, 2) significant hepatic or renal dysfunction (creatinine >150 mmol/L), 3) signifi- cant pulmonary or thyroid disease, 4) any connective tissue disease or history of malignancy, 5) episode of recent heart failure, 6) acute coronary syndrome within the last 3 months and, 7) smoking within the previous 3 months. The present study was approved by the local ethical committee of Laval Hospital. A sample of right auricular appendix was taken at the beginning of the CABG procedure, and immediately placed in an oxygenated Tyrode solution (in mM: NaCl 137, KCl 5.4, CaCl2 1.8, MgCl2 1, NaHCO3 12, NaH2PO4 0.4, glucose 5.6) at 4℃. The tissue was sub- sequently immersed in liquid nitrogen and stored at -80 ℃ for western analyses. 2.2. Heart Rate Variability Heart rate variability (HRV) was derived from a 24-hour Holter monitoring system (Marquette Electronics Inc., Milwaukee, WI) in all subjects during normal daily life activity. HRV derived from 24-hour ambulatory moni- toring is reproducible and free of placebo effect [21]. Using frequency domains, power in the low frequency (LF, 0.04-0.15 Hz) that is an index of both sympathetic and parasympathetic activity, and high frequency (HF, 0.15-0.4 Hz) that is an index of solely parasympathetic activity, were calculated. LF/HF ratio is the power in low frequency divided by the power in high frequency. Using time domains, the standard deviation of the RR intervals (SDNN), the square root of the mean squared differences of successive RR intervals (rMSSD) and the standard deviation of the average RR intervals calculated over 5-min periods (SDANN) were determined. pNN50 is the proportion of interval differences of successive NN intervals greater than 50 ms. NN intervals are the normal-to-normal intervals that include all intervals be- tween adjacent QRS complexes resulting from sinus node depolarizations in the entire 24-hour ECG re- cording as previously reported [7]. 2.3. Protein Isolation and Western Blot Analysis [22,23] Total protein content was determined with bovine serum albumin as standard and subsequently separated with an 8% denaturing-PAGE. Proteins transferred to Immobilon PVDF membranes were incubated with antibodies for Kv1.5 [Alomone Labs], Cx40 [Chemicon Int.] (poly- clonal, raised in rabbit) and Cx43 [Chemicon Int.] (monoclonal, mouse). Epitopes are residues 513-602 of mouse Kv1.5, gap junction alpha-5 protein of mouse connexin 40 [CxA-5], and residues 252-270 of native sequence from rat cardiac connexin 43, respectively. All primary antibodies specific for these 3 proteins were diluted in TBS-Tween 1:200. Membranes were respec- tively incubated with secondary antibodies (1:10000 and 1:5000, anti-rabbit [Cedarlane]; 1:100000, anti-mouse [Chemicon Int.]), conjugated with peroxidase. An ECL detection kit [Millipore] was used to reveal the anti- gen-antibody for subsequent densitometric analysis. Kv1.5, Cx43 and Cx40 protein levels were normalized to α-tubulin level (1:250, polyclonal, raised in rabbit, Ab- cam). 2.4. Statistical Analysis Patient characteristics, Holter and blood chemistry pa- rameters are reported as mean ± standard deviation unless otherwise specified (S.D.). Densitometry values are expressed as mean ± S.E.M. Comparisons between 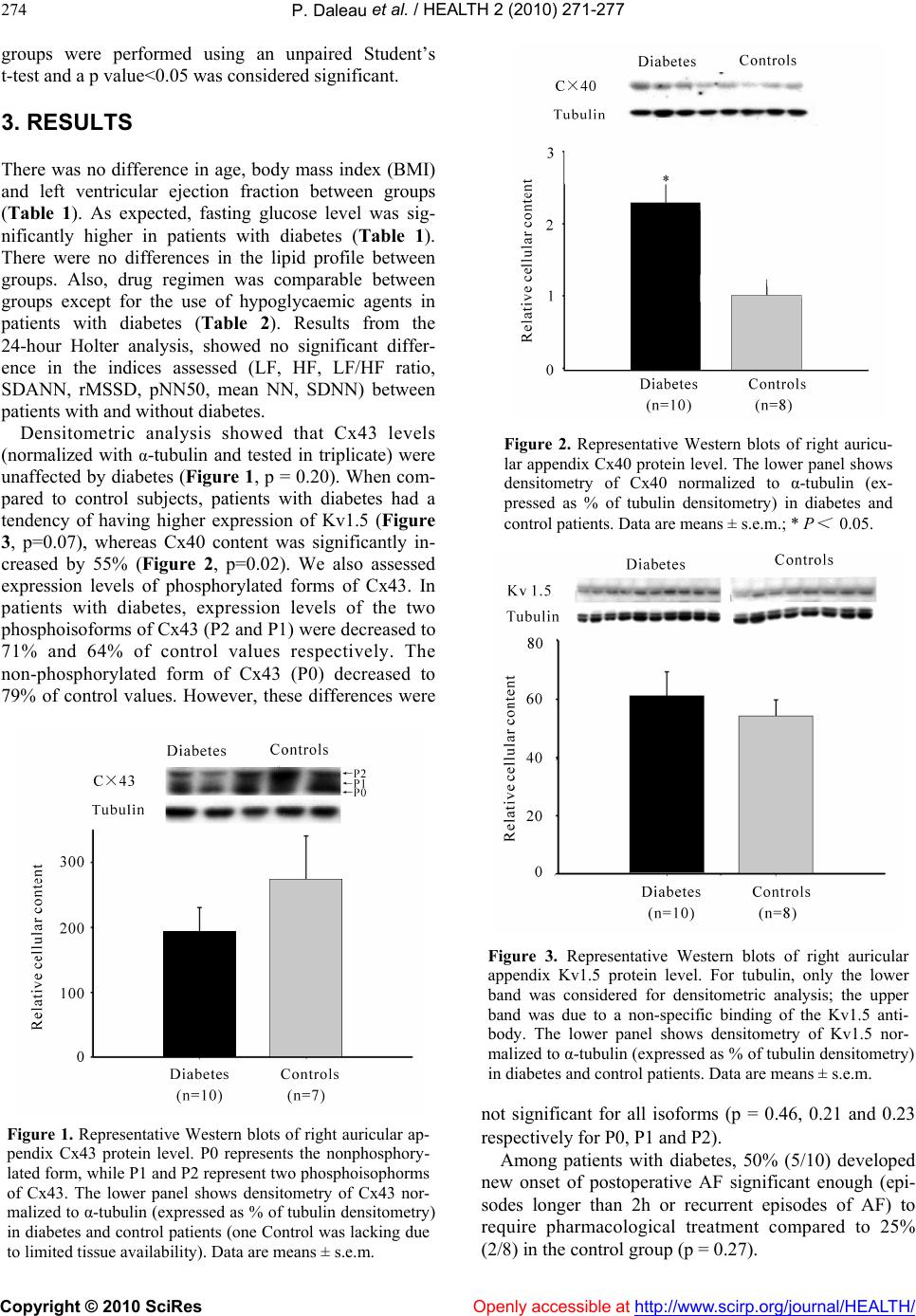 P. Daleau et al. / HEALTH 2 (2010) 271-277 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 274 groups were performed using an unpaired Student’s t-test and a p value<0.05 was considered significant. 3. RESULTS There was no difference in age, body mass index (BMI) and left ventricular ejection fraction between groups (Table 1). As expected, fasting glucose level was sig- nificantly higher in patients with diabetes (Table 1). There were no differences in the lipid profile between groups. Also, drug regimen was comparable between groups except for the use of hypoglycaemic agents in patients with diabetes (Table 2). Results from the 24-hour Holter analysis, showed no significant differ- ence in the indices assessed (LF, HF, LF/HF ratio, SDANN, rMSSD, pNN50, mean NN, SDNN) between patients with and without diabetes. Densitometric analysis showed that Cx43 levels (normalized with α-tubulin and tested in triplicate) were unaffected by diabetes (Figure 1, p = 0.20). When com- pared to control subjects, patients with diabetes had a tendency of having higher expression of Kv1.5 (Figure 3, p=0.07), whereas Cx40 content was significantly in- creased by 55% (Figure 2, p=0.02). We also assessed expression levels of phosphorylated forms of Cx43. In patients with diabetes, expression levels of the two phosphoisoforms of Cx43 (P2 and P1) were decreased to 71% and 64% of control values respectively. The non-phosphorylated form of Cx43 (P0) decreased to 79% of control values. However, these differences were Figure 1. Representative Western blots of right auricular ap- pendix Cx43 protein level. P0 represents the nonphosphory- lated form, while P1 and P2 represent two phosphoisophorms of Cx43. The lower panel shows densitometry of Cx43 nor- malized to α-tubulin (expressed as % of tubulin densitometry) in diabetes and control patients (one Control was lacking due to limited tissue availability). Data are means ± s.e.m. Figure 2. Representative Western blots of right auricu- lar appendix Cx40 protein level. The lower panel shows densitometry of Cx40 normalized to α-tubulin (ex- pressed as % of tubulin densitometry) in diabetes and control patients. Data are means ± s.e.m.; * P < 0.05. Figure 3. Representative Western blots of right auricular appendix Kv1.5 protein level. For tubulin, only the lower band was considered for densitometric analysis; the upper band was due to a non-specific binding of the Kv1.5 anti- body. The lower panel shows densitometry of Kv1.5 nor- malized to α-tubulin (expressed as % of tubulin densitometry) in diabetes and control patients. Data are means ± s.e.m. not significant for all isoforms (p = 0.46, 0.21 and 0.23 respectively for P0, P1 and P2). Among patients with diabetes, 50% (5/10) developed new onset of postoperative AF significant enough (epi- sodes longer than 2h or recurrent episodes of AF) to require pharmacological treatment compared to 25% (2/8) in the control group (p = 0.27).  P. Daleau et al. / HEALTH 2 (2010) 271-277 Copyright © 2010 SciRes http://www.scirp.org/journal/HEALTH/Openly accessible at 275 Table 1. Patient characteristics. Diabetes (n=10) Controls (n=8) P value Age (years) BMI (kg/m2) EF (%) Men/Women 61.5 ± 13.1 34.1 ± 7.5 57.3 ± 7.1 7/3 68.4 ± 6.7 29.5 ± 4.4 63.4 ± 8.7 7/1 0.197 0.198 0.065 0.815 Blood analysis: Glucose (mmol/L) HbA1c (%) Apo B (g/L) 7.2 ± 1.6 7.1 ± 1.0 0.71 ± 0.18 5.2 ± 0.6 - 0.86 ± 0.21 0.003 - 0.148 Lipid profile: Total cholesterol (mmol/L) Triglycerides (mmol/L) HDL cholesterol (mmol/L) LDL cholesterol (mmol/L) Total-C/HDL-C 3.54 ± 0.62 1.72 ± 0.87 1.11 ± 0.14 1.65 ± 0.48 3.20 ± 0.58 4.04 ± 0.81 1.82 ± 0.99 1.18 ± 0.27 2.03 ± 0.50 3.57 ± 1.08 0.160 0.756 0.657 0.120 0.372 BMI = Body mass index; EF = Ejection fraction Table 2. Co-morbidities and drug regimen in both groups. Diabetes % (n) (n=10) Controls % (n) (n=8) P value History Hypertension Dyslipidemia Smoking 70 (7) 70 (7) 10 (1) 62.5 (5) 75 (6) 37.5 (3) 0.107 1.000 1.000 Rx Classes Nitroglycerin β-blockers CCB ACEI ARB α1 inhibitors ASA Diuretics Statins Fibrates Oral hypoglycemic agents Benzodiazepine Antidepressants 40 (4) 90 (9) 50 (5) 30 (3) 60 (6) 10 (1) 90 (9) 40 (4) 90 (9) - 100 (10) 10 (1) 10 (1) 50 (4) 87.5 (7) 50 (4) - 12.5 (1) 12.5 (1) 87.5 (7) - 62.5 (5) 12.5 (1) - 25 (2) - 0.143 1.000 1.000 - 1.000 1.000 0.125 - 0.375 - - 1.000 - 4. DISCUSSION This study assessed the effects of type 2 diabetes on the expressions of connexins and Kv1.5 in the human heart. The most important finding of this work is that patients with type 2 diabetes had significantly higher expression of Cx40 in the right auricular appendix tissue. Apart from the diabetic status, the 2 groups were well matched in terms of age and risk factors, implying that the ob- served modulation of connexin is likely to reflect the effect of diabetes per se. 4.1. Expression of Connexins and Modulation by Diabetes Assessment of protein content revealed that when com- pared to control patients, the level of Cx40 in the right auricular appendix specimens was increased by 55 % in individuals having type 2 diabetes. Similarly, a recent study in rats with streptozotocin-induced diabetes has shown that expression of Cx40 was increased in retinal microvessels [18]. Although not statistically significant, we also observed a trend toward a decreased expression of Cx43 in the right auricular appendix tissues of pa-  P. Daleau et al. / HEALTH 2 (2010) 271-277 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 276 tients with type 2 diabetes. All isoforms of Cx43 (the two phosphoisoforms and the non-phosphorylated form), were diminished, although non-significantly. It should be emphasized that in diabetic animal models a reduc- tion in the expression of Cx43 has been documented in several tissues [17,24,25], including the heart [18,26], though exceptions also exist [27,28]. In one study, while a down-regulation of Cx43 was demonstrated in retinal microvessels of diabetic rats, an over-expression of Cx40 and Cx45 were found [18]. Thus, it seems possible that a down-regulation of one type of connexin is offset by the increased production of another type of gap junction protein. In the same line, a recent study analyzing the expression of connexins in the bladder of diabetic rat reported a lower amount of Cx43 isoform and higher expression of Cx32 and 26 [17]. However, it has been stressed that the function of one type of connexin cannot necessarily be restored by the presence of another but different member of the con- nexin family [29]. Hence, this ‘replacement’ hypothesis certainly deserves more investigations. 4.2. Atrial Fibrillation, Connexins and K+ Channels Diabetes has been reported as a strong and independent risk factor for the development of AF [30,31]. Of note, episodes of postoperative AF have been associated to a higher expression Cx40 in the right auricular appendix obtained from patients undergoing cardiac surgery [32, 33,34,35]. It is likely that the relative abundance of Cx43 and Cx40 plays an important role in the atrial impulse propagation, and whereby dominance of Cx40 decreases local propagation velocity [36]. Therefore, the modula- tion of Cx40 expression by the diabetic environment could lead to micro-heterogeneities of electrical conduc- tion pattern, promoting by this way episodes of AF. In different studies with experimental animal models, the activity of several cardiac potassium currents, such as Ito, IK and Iss, are modulated by diabetes and are possibly involved in the development and/or maintenance of AF [37,38,39]. However, the present results in human right auricular ap- pendix tissue show that Kv1.5 expression remains un- changed in patients with diabetes, suggesting that IKur is not affected. Nevertheless, one cannot exclude a direct modula- tion of this current by glucose and/or insulin levels. 4.3. Clinical Implications AF is the most frequent arrhythmia and has been linked to aging as well as to diabetes. While atrial remodeling is undoubtedly an important process participating to the development and the maintenance of AF, an electro- physiological substrate is actively involved in the genera- tion of arrhythmia. In this regard, the dispersion of atrial refractoriness and the ensuing development of multiple reentry wavelets are likely to be important underlying mechanisms pertaining to AF. In this study, the finding of a significant modulation of Cx40 in patients with diabetes may partly explain the well-documented association be- tween diabetes and AF. By which mechanism diabetes contributes to increase Cx40 expression in atrial tissue is still unknown, but it is possible that inflammatory and/or oxidative stress pathways [40, 41], which are incidentally activated in subjects with diabetes, may contribute to modify the amount of connexins in atrial cardiomyocytes. 4.4. Limitations Insofar patients in the present study had significant coronary artery disease and other co-morbidities, the present findings cannot be inferred to the overall spec- trum of patients with diabetes. Particularly, pre-diabetic subjects with glucose intolerance were not studied and may have given further insights as to whether the ob- served modifications may occur at an earlier stage. In addition, the size of the left atria, which represent atrial remodeling, was not documented in the present study. However, the effect of atrial remodeling, a possible confounding variable, was minimized by having selected a cohort of patients without significant valve disease, a normal ejection fraction, and in sinus rhythm. 4.5. Conclusions In conclusion, we found an up-regulation of Cx40 pro- tein content in right auricular appendix tissue from pa- tients with type 2 diabetes. In light of other studies hav- ing demonstrated a link between AF and Cx40 expres- sion, it is possible that higher prevalence of AF in pa- tients with diabetes is explained, at least partially, by differential expression of gap-junction proteins. Albeit further studies are still needed to understand the full im- plication of Cx40 in AF, this study casts some light on a potentially important process occurring in individuals with diabetes, and may open-up new therapeutic and/or research vistas in order to treat and/or prevent AF in this at-risk population. 5. ACKNOWLEDGEMENTS Drs Paul Poirier and Patrick Mathieu are research scholars from the Fonds de la Recherche en Santé du Québec. This work was supported by operating grants from the Quebec Heart Institute (Drs Paul Poirier and Pascal Daleau) and the Heart and Stroke Foundation of Quebec (Dr Pascal Daleau). REFERENCES [1] Gu, K., Cowie, C.C. and Harris, M.I. (1999) Diabetes and decline in heart disease mortality in US adults. Jour- nal of the American Medical association, 281, 1291- 1297.  P. Daleau et al. / HEALTH 2 (2010) 271-277 Copyright © 2010 SciRes Openly accessible at http://www.scirp.org/journal/HEALTH/ 277 [2] Grundy, S.M., Benjamin, I.J., Burke, G.L., Chait, A., Eckel, R.H., Howard, B.V., Mitch, W., Smith, S.C.Jr. and Sowers, J.R. (1999) Diabetes and cardiovascular disease: a statement for healthcare professionals from the American Heart Association. Circulation, 100, 1134- 1146. [3] Fuster, V., Ryden, L.E., Cannom, D.S., Crijns, H.J., Cur- tis, A.B., Ellenbogen, K.A., Halperin, J.L., Le Heuzey, J.-Y., Kay, G.N., Lowe, J.E., Olsson, S.B., Prystowsky, E.N., Tamargo, J.L. and Wann, S. (2006) ACC/AHA Task Force Members, ESC Committee for Practice Guidelines: ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the European Society of Cardiology Committee for Practice Guidelines (Writing Committee to revise the 2001 guide- lines for the management of patients with atrial fibrilla- tion) developed in collaboration with the European Heart Rhythm Association and the Heart Rhythm Society. Circulation, 114, e257-e354. [4] Benjamin, E.J., Levy, D., Vaziri, S.M., D'Agostino, R.B., Belanger, A.J. and Wolf, P.A. (1994) Independent risk factors for atrial fibrillation in a population-based cohort. The Framingham Heart Study. Journal of the American Medical association, 271, 1-11. [5] Allessie, M.A., Boyden, P.A., Camm, A.J., Kléber, A.G., Lab, M.J., Legato, M.J., Rosen, M.R., Schwartz, P.J., Spooner, P.M., Van Wagoner, D.R. and Waldo, A.L. (2001) Pathophysiology and prevention of atrial fibrilla- tion. Circulation, 103, 769-777. [6] Poirier, P., Bogaty, P., Garneau, C., Marois, L. and Du- mesnil, J.G. (2001) Diastolic dysfunction in normoten- sive men with well-controlled type 2 diabetes: impor- tance of maneuvers in echocardiographic screening for preclinical diabetic cardiomyopathy. Diabetes Care, 24, 5-10. [7] Poirier, P., Bogaty, P., Philippon, F., Garneau, C., Fortin, C. and Dumesnil, J.G. (2003) Preclinical diabetic car- diomyopathy: relation of left ventricular diastolic dys- function to cardiac autonomic neuropathy in men with uncomplicated well-controlled type 2 diabetes. Metabo- lism, 52, 1056-1061. [8] Magyar, J., Rusznak, Z., Szentesi, P., Szucs, G. and Kovacs, L. (1992) Action potentials and potassium cur- rents in rat ventricular muscle during experimental dia- betes. Journal of Molecular and Cellular Cardiology, 24, 841-853. [9] Nishiyama, A., Ishii, D.N., Backx,.P.H., Pulford, B.E., Birks, B.R. and Tamkun, M.M. (2001) Altered K+ chan- nel gene expression in diabetic rat ventricle: isoform switching between Kv4.2 and Kv1.4. American Journal of Physiology, 281, H1800-H1807. [10] Gallego, M., Casis, E. and Izquierdo, M.J. (2000) Resto- ration of cardiac transient outward potassium current by norepinephrine in diabetic rats. Pflügers Archives, 441, 102-107. [11] Xu, Z., Patel, K., Lou, M.F. and Rozanski, G.J. (2002) Up-regulation of K+ channels in diabetic rat ventricular myocytes by insulin and glutathione. Cardiovascular Research, 53, 80-88. [12] Pacher, P., Ungvari, Z., Nanasi, P.P. and Kecskeméti, V. (1999) Electrophysiological changes in rat ventricular and atrial myocardium at different stages of experimental diabetes. Acta Physiologica Scandinavica, 166, 7-13. [13] Yang, Q., Kiyoshige, K., Fujimoto, T., Katayama, M., Fujino, K., Saito, K., Nakaya, Y. and Mori, H. (1990) Signal-averaging electrocardiogram in patients with dia- betes mellitus. Japanese Heart Journal, 31, 25-33. [14] Celiker, A., Akinci, A. and Özin, B. (1994) The sig- nal-averaged electrocardiogram in diabetic children. In- ternational Journal of Cardiology, 44, 271-274. [15] Inoguchi, T., Ueda, F., Umeda, F., Yamashita, T. and Nawata, H. (1995) Inhibition of intercellular communi- cation via gap junction in cultured aortic endothelial cells by elevated glucose and phorbol ester. Biochemical and Biophysical Research Communications, 208, 492-497. [16] Kuroki, T., Inoguchi, T., Umeda, F., Ueda, F. and Nawata, H. (1998) High glucose induces alteration of gap junction permeability and phosphorylation of con- nexin-43 in cultured aortic smooth muscle cells. Diabetes, 47, 931-936. [17] Inoguchi, T., Yu, H.Y., Imamura, M., Kakimoto, M., Kuroki, T., Maruyama, T. and Nawata, H. (2001) Altered gap junction activity in cardiovascular tissues of diabetes. Medical Electron Microscopy, 34, 86-91. [18] Oku, H., Kodama, T., Sakagami, K., Puro, D.G. (2001) Diabetes-induced disruption of gap junction pathways within the retinal microvasculature. Investigative Oph- thalmology and Visual Science, 42, 1915-1920. [19] Poladia, D.P., Schanbacher, B., Wallace, L.J. and Bauer, J.A. (2005) Innervation and connexin isoform expression during diabetes-related bladder dysfunction: early struc- tural vs. neuronal remodeling. Acta Diabetologica, 42, 147-152. [20] Stilli, D, Lagrasta, C., Berni, R., Bocchi, L., Savi, M., Delucchi, F., Graiani, G., Monica, M., Maestri, R., Ba- ruffi, S., Rossi, S., Macchi, E., Musso, E. and Quaini, F. (2007) Preservation of ventricular performance at early stages of diabetic cardiomyopathy involves changes in myocyte size, number and intercellular coupling. Basic Research in Cardiology, 102, 488-499. [21] Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysi- ology: Heart rate variability (1996) Standards of meas- urement, physiological interpretation, and clinical use. European Heart Journal, 17, 354-381. [22] Daleau, P., Boudriau, S., Michaud, M., Jolicoeur, C. and Kingma, J.G. Jr. (2001) Preconditioning in the absence or presence of sustained ischemia modulates myocardial Cx43 protein levels and gap junction distribution. Cana- dian Journal of Physiology and Pharmacology, 79, 371- 378. [23] Sarrazin, J.-F., Comeau, G., Daleau, P., Kingma, J., Plante, I., Fournier, D. and Molin, F. (2007) Reduced in- cidence of vagally-induced atrial fibrillation and expres- sion levels of connexins by n-3 polyunsaturated fatty ac- ids in dogs. Journal of the American College of Cardiol- ogy, 50, 1505-1512. [24] Lin, H., Ogawa, K., Imanaga, I. and Tribulova, N. (2006) Alterations of connexin 43 in the diabetic rat heart. Ad- vance in Cardiology, 42, 243-254. [25] Okruhlicova, L., Tribulova, N., Misejkova, M., Kucka, M., Stetka, R., Slezak, J. and Manoach, M. (2002) Gap  P. Daleau et al. / HEALTH 2 (2010) 271-277 Copyright © 2010 SciRes http://www.scirp.org/journal/HEALTH/Openly accessible at 278 junction remodeling is involved in the susceptibility of diabetic rats to hypokalemia-induced ventricular fibrilla- tion. Acta Histochemica, 104, 387-391. [26] Sheu, J.-J., Chang, L.-T., Chiang, C.-H., Sun, C.-K., Chang, N.-K., Youssef, A.A., Wu, C.-J., Lee, F.-Y. and Yip, H.-K. (2007) Impact of diabetes on cardiomyocyte apoptosis and connexin43 gap junction integrity. Role of pharmacological modulation. International Heart Jour- nal, 48, 233-245. [27] Brink, P.R., Valiunas, V., Wang, H.Z., Zhao, W., Davies, K. and Christ, G.J. (2006) Experimental diabetes alters connexin43 derived gap junction permeability in short- term cultures of rat corporeal vascular smooth muscle cells. Journal of Urology, 175, 381-386. [28] Howarth, F., Nowotny, N., Zilahi, E., El Haj, M. and Lei, M. (2007) Altered expression of gap junction connexin proteins may partly underlie heart rhythm disturbances in the streptozotocin-induced diabetic rat heart. Molecular and Cellular Biochemistry, 305, 145-151. [29] Wolfle, S.E., Schmidt, V.J., Hoepfl, B., Gebert, A., Al- colea, S., Gros, D. and de Wit, C. (2007) Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circu- lation Research, 101, 1292-1299. [30] Östgren, C.J., Merlo, J., Råstam, L. and Lindblad, U. (2004) Atrial fibrillation and its association with type 2 diabetes and hypertension in a Swedish community. Diabetes, Obesity and Metabolism, 6, 367-374. [31] Movahed, M.-R., Hashemzadeh, M. and Jamal, M.M. (2005) Diabetes mellitus is a strong, independent risk for atrial fibrillation and flutter in addition to other cardio- vascular disease. International Journal of Cardiology, 105, 315-318. [32] Dupont, E., Ko, Y.-S., Rothery, S., Coppen, S.R., Baghai, M., Haw, M. and Severs, N.J. (2001) The gap-junctional protein connexin 40 is elevated in patients susceptible to postoperative atrial fibrillation. Circulation, 103, 842- 849. [33] Polontchouk, L., Haefliger, J.-A., Ebelt, B., Schaefer, T., Stuhlmann, D., Mehlhorn, U. and Kuhn-Regnier, F. (2001) Effects of chronic atrial fibrillation on gap junc- tion distribution in human and rat atria. Journal of the American College of Cardiology, 38, 883-891. [34] Kostin, S., Klein, G., Szalay, Z., Hein, S., Bauer, E.P. and Schaper, J. (2002) Structural correlate of an atrial fibrillation in human patients. Cardiovascular Research, 54, 361-379. [35] Wetzel, U., Boldt, A., Lauschke, J., Weigl, J., Schirde- wahn, P., Dorszewski, A., Doll, N., Hindricks, G., Dhein, S. and Kottkamp, H. (2005) Expression of connexins 40 and 43 in human left atrium in atrial fibrillation of dif- ferent aetiologies. Heart, 91, 166-170. [36] Beauchamp, P., Yamada, K.A., Baertschi, A.J., Green, K., Kanter, E.M., Saffitz, J.E. and Kléber, A.G. (2006) Relative contributions of connexins 40 and 43 to atrial impulse propagation in synthetic strands of neonatal and fetal murine cardiomyocytes. Circulation Research, 99, 1216-1224. [37] Zhang, Y., Xiao, J., Lin, H., Luo, X., Wang, H., Bai, Y., Wang, J., Zhang, H., Yang, B. and Wang, Z. (2007) Ionic mechanisms underlying abnormal QT prolongation and the associated arrhythmias in diabetic rabbits: a role of rapid delayed rectifier K+ current. Cellular Physiology and Biochemistry, 19, 225-238. [38] Lengyel, C., Virag, L., Biro, T., Jost, N., Magyar, J., Biliczki, P., Kocsis, E., Skoumal, R., Nanasi, P.P., Toth, M., Kecskemeti, V., Papp, J.G. and Varro, A. (2007) Diabetes mellitus attenuates the repolarization reserve in mammalian heart. Cardiovascular Research, 73, 512- 520. [39] Casis, O., Gallego, M., Iriarte, M. and Sanchez-Chapula, J.A. (2000) Effects of diabetic cardiomyopathy on re- gional electrophysiologic characteristics of rat ventricle. Diabetologia, 43, 101-109. [40] Fernandez-Cobo, M., Gingalewski, C., Drujan, D. and De Maio, A. (1999) Downregulation of connexin 43 gene expression in rat heart during inflammation. The role of tumour necrosis factor. Cytokine, 11, 216-224. [41] Fukuda, T., Ikejima, K., Hirose, M., Takei, Y., Watanabe, S. and Sato, N. (2000) Taurine preserves gap junctional intercellular communication in rat hepatocytes under oxidative stress. Journal of Gastroenterology, 35, 361- 368.
|