Photokilling of Escherichia coli Using Hybrid Titania Nanoparticles Suspended in an Aqueous Liquid ()
1. Introduction
New technologies for disinfection applications are a growing market nowadays. Alternative process to conventional disinfection methods are required to meet the new requirements of health care regulations in numerous applications. Concerning the water disinfection, the use of free chlorine as a disinfectant of ground water containing a high rate of organic species induces the genesis of disinfection by-products (DBS) hazardous to human health [1-3]. Medical applications also require high efficiency and none hazardous sterilization process. Surgery devices surfaces are colonized by bacteria [4-6]: the emergence of resistant and virulent strains of micro-organism combined to the wide spread use of antibiotics necessitate new disinfection technologies. Disinfection of areas of intensive medical use or laboratories surfaces is essential for the biological security [7]. The manual disinfection by wiping using aggressive chemicals is not efficient enough and may cause troubles to the people. The use of TiO2 nanoparticles in suspension as a photocatalytic disinfection device seems to be an interesting alternative to conventional process. No expensive or dangerous chemicals are needed, and high disinfection efficiency can be reached, combined to the mineralization of the organic species. Many studies have reported the efficiency of photocatalysis for destroying microorganisms in water. Extensive research has been done about the destruction of E. coli using TiO2 suspension and TiO2 films [8-12]. This bactericidal effect of nanoparticles was explained either by disruption of cell membrane activity [13,14] or induction of intercellular reactive oxygen species, including hydrogen peroxide (H2O2), superoxide (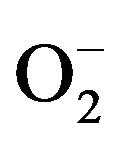 ) and hydroxyl radical (HO•) strong oxidizing agents harmful to bacterial cells [13,15]. In this study, we investigate both the physico-chemical properties of our synthesized suspension of anatase crystallized TiO2 nanoparticles and the inactivation of Gram-negative Escherichia coli (strain LE392) by a photokilling process.
) and hydroxyl radical (HO•) strong oxidizing agents harmful to bacterial cells [13,15]. In this study, we investigate both the physico-chemical properties of our synthesized suspension of anatase crystallized TiO2 nanoparticles and the inactivation of Gram-negative Escherichia coli (strain LE392) by a photokilling process.
2. Materials and Methods
2.1. Nanoparticles Suspension Synthesis
The precursor solution was 10 mL titanium IV isopropoxide 97% supplied by ©Sigma Aldrich mixed with 10 mL of anhydrous isopropanol (from ©Sigma) under a vigorous stirring. The titanium alkoxide reactivity is lowered by the use of acetylacetone as chelating reagent. The spontaneous hydrolysis of the titanium isopropoxide is obtained by the quick addition of 75 mL of acidified water. The reacting medium is then heated to 80˚C under reflux for almost 8 hours (peptidization process). After this step, the dispersion is cooled down to room temperature. A clear, yellow, nanoparticles suspension is obtained.
2.2. X-Ray Diffraction Analysis
XRD measurements are performed on the ©Philips x’pert MPD diffractometer operating in the reflection mode with Cu-Kα1 radiation (1.5406 Å, 40 kV, 55 mA) and diffracted beam monochromator, using a step scan mode with a step of 0.1˚ (2θ) and 3 s per step. Diffraction pattern obtained from the TiO2 nanopowder is compared with reference to the Join Committee on Powder Diffraction Standards (JCPDS) database.
2.3. Transmission Electron Microscopy
The morphology and the particles sizes were characterized using a ©Philips CM 20 transmission electron microscope (TEM). The accelerating voltage was 200 kV. The samples were dispersed in methanol by ultra sonication. A drop of the suspension was then laid on a carboncoated grid and dried. Selected Area Electron Diffraction (SAED) was performed to determine the crystallinity of the structure. The interplanar spacings were measured from the SAED patterns using Equation (1):
 (1)
(1)
where λL is a constant of the microscope, R is the ring radius, and d is the interplanar distance. The constant of the microscope was calculated by measuring the radius of a gold standard pattern which interplanar distances are well documented in literature.
2.4. Thermogravimetric Analysis
The thermogravimetric analysis was carried out using the TGA 4000 thermogravimetric analyser (©Perkin Elmer instrument). The analysis was performed at a heating rate of 5˚C·min−1 under a 40 mL·min−1 nitrogen gas flow. Prior to the TGA tests, a designated volume of 20 mL of the TiO2 suspension was dried at 110˚C overnight.
2.5. Photocatalytic Activity Study
Photodegradation of an organic dye, Acid Orange 7 supplied by ©Acros-Organic (AO7) in aqueous solution was used as a probe to assess the photocatalytic activity of the TiO2 suspension.
The experiments were carried out using 3 mL of AO7 solution (5×10−5 mol·L−1). In order to avoid the dilution effect, 60 µL of the TiO2 suspension was added to the 3 mL of the organic dye solution under stirring at 200 rpm. After homogenization, the mixture was irradiated in an air-cooled cylindrical reactor under stirring with polychromatic fluorescent UV lamps (©Philips TLD 8W) providing a total power of 48 W, in a configuration delivering about 0.8 mW·cm−2 at the liquid surface. Each sample was irradiated one time and replaced by a new one to avoid cumulative effect.
The UV induced degradation of the organic dye was recorded from 200 to 700 nm with a resolution of 2 nm using a ©Perkin Elmer Lambda 35 spectrometer. Quartz glass cells with an optical pathway of 1 cm were used. De-ionized water was taken as reference. The reaction was followed by monitoring the decrease of the solution’s absorbance at 483 nm (strong absorption band of the Acid Orange 7).
2.6. Bacterial Culture
Escherichia coli LE392 was used as a model micro-organism for photokilling experiments. Bacteria cells were cultured at 37˚C for 12 h in Nutrient Broth medium at pH 7.2 (Biokar Diagnostics) containing Tryptone (10 g·L−1), Meat extract (5 g·L−1) and Sodium Chloride (5 g·L−1) after 12 h of pre-culture in the same conditions. Cells were centrifuged at 2500 g for 15 min at 4˚C and the pellet was re-suspended in de-ionized water to prevent unintentional increase in cell numbers. The initial population of E. coli was determined by enumeration with a Petroff-Hausser Counting Chamber.
2.7. Photokilling Measurements
An amount of 20 ml of de-ionized water was inoculated with E. coli suspension in order to achieve a concentration of 106 cfu·mL−1. This culture was placed in a Petri plate and TiO2 nanoparticles, either in nanopowder form or suspended in the carrying liquid, were added to achieve a final concentration in TiO2 of 1 g·L−1. The slurries were continuously mixed with a sterilized Teflon magnetic stir bar placed in the Petri dish with a speed of 200 rpm to allow a complete mixing.
In order to investigate the potential toxicity of the carrying liquid in which the TiO2 nanoparticles were suspended, measurements were performed on the mixture composed of E. coli suspension and the designed volume of carrying liquid without TiO2 nanoparticles. Chemical composition of the carrying liquid is described in Table 1.
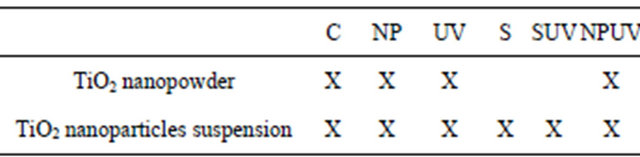
All experiments were made in Petri dishes and the different experimental conditions are presented in Table 2.
Irradiation was provided by polychromatic fluorescent UV lamps (©Philips TLD 8W) providing a total power of 48 W, in a configuration delivering 1.5 mW·cm−2 at
Table 1. Chemical composition of the carrying liquid in which the TiO2 nanoparticles are suspended.

Table 2. Different assays realized with the TiO2 nanopowder from the synthesis and the synthesized TiO2 nanoparticles suspension. C = E. coli suspension in the dark; NP = E. coli suspension in presence of the TiO2 nanopowder from the synthesis or synthesized TiO2 nanoparticles suspension. UV = E. coli irradiated with UV light; S = E. coli suspension with the carrying liquid; SUV = E. coli suspension irradiated with UV light in presence of the carrying liquid; NPUV = E. coli suspension irradiated with UV light in presence of nanoparticles.
the liquid surface. Sampling of the solutions was done at requisite time intervals by pipetting 1 mL from the suspension and serially diluted in 9 mL of Ringer’s solution. After well mixing, 100 µL aliquots of each dilution were plated onto solid Nutrient Gelose medium (Agar 1.5 g·L−1). Colony-forming units were counted after overnight incubation at 37˚C. All experiments were made in aseptic conditions to prevent any contamination in mediums. The counts from three independent experiments corresponding to a particular sample were averaged.
3. Results and Discussions
3.1. Titania Nanoparticle Synthesis
Crystalized TiO2 nanoparticles in suspension were successfully synthesized by chelation and hydrolysis-condensation of the titanium alkoxide precursor. The synthesis included a low temperature protocol which is a significant advantage compared to the others requiring high temperature to obtain crystalized phase [16,17]. Another advantage is that the stable suspension obtained avoids the use of potentially toxic dry nanopowder. Studies are dealing with the research of the suspensions’ stability and their biological behavior [18]. The process followed induces high stability of the suspension and opens the way to develop interesting photocatalytic process. The chemical functionalization of the titanium alkoxide by a beta diketone is a substitution of alkoxy group by a less hydrolysable one such as acetylacetone. This chelation slows down the hydrolysis kinetic, avoids undesired precipitate and promotes the dispersion of the colloids in the liquid.
3.2. X-Ray Diffraction on the TiO2 Nanopowder
X-ray diffraction of the dried nanopowder is shown in Figure 1. XRD pattern exhibits strong diffraction peaks at 25˚C and 38˚C indicating TiO2 in the anatase phase. All peaks present are in good agreement with the standards spectrum (JCPDS n.: 21-1272). The crystallite size, D, is evaluated by measuring the width of the curves produced and using the Scherrer formula (2):
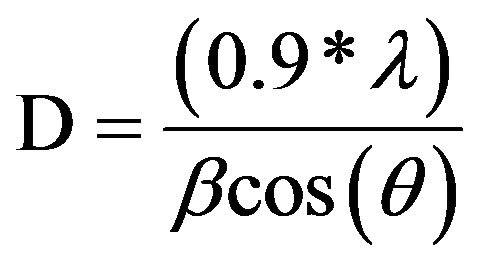 (2)
(2)
where λ is the wavelength of the Cu K α1 line in nm, β is the full-width at half max (FWHM) of the peak. Using the FWHM of the (101) anatase peak, we found that the average crystal size was about 8 nm.
3.3. Transmission Electron Microscopy
A typical TEM image of the as-synthesized TiO2 nanoparticles is presented in Figure 2(a). The particles are flake-shaped and tend to agglomerate to form aggregates but less than reported in the case of nanopowder [19]. This well-dispersed situation is attributed to the chemical functionalization of the surface which acts as surfactant and promotes the individualization of the particles. Generally speaking, the agglomeration tendency is explained by the fact that the new state is more stable in the energetic point of view and allows the crystallite growth. The different TEM images of our study are consistent with a well-dispersed suspension of nanoparticles. From these pictures, the crystallite size is between 7 and 20 nm. The SAED pattern of the nanoparticles is shown in Figure 2(b). A comparison between the interplanar distances calculated from the SAED pattern and the tabulated ones for the TiO2 anatase crystallographic structure
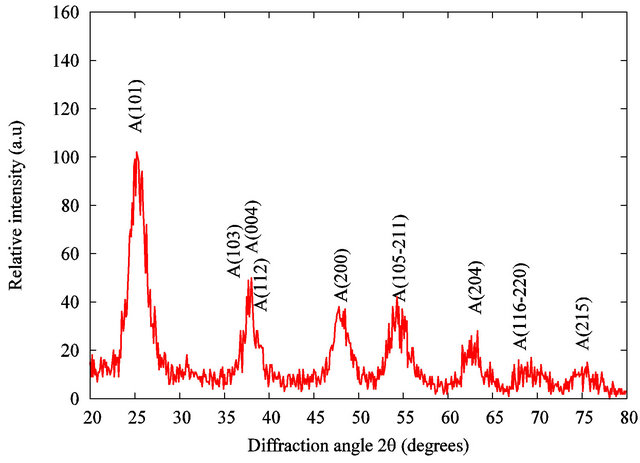
Figure 1. X-ray diffraction of the TiO2 grafted acetylacetone nanopowder.
reveals a high degree of consistency (Table 3). This comparison reveals the polycrystalline nature of our sample. The interplanar spacings measured correspond to the TiO2 anatase structure. This study confirms the results obtained by the XRD analysis on the nanopowder and validates our synthesis approach: a stable suspension of TiO2 anatase nanoparticles in aqueous medium is obtained.
3.4. Thermogravimetry Study
Table 4 gives the characteristics of the samples analysed by TGA. Sample S2 was used as a reference for TiO2 nanopowder without organic components. Sample S1 was the organic functionalized TiO2 nanopowder from the suspension. TGA measurements were carried out from 50˚C to 1000˚C at a heating rate of 5˚C·min−1.
Figure 3 shows the TGA curves.
As far as the manufactured TiO2 nanopowder is concerned, no significant weight loss is recorded in the 100˚C - 1000˚C range. In comparison, the weight loss curve of the organic functionalized TiO2 nanopowder exhibits two significant weight losses. The first one occurs in the 100˚C - 200˚C range and is attributed to the desorption of volatile compound such as residual water and
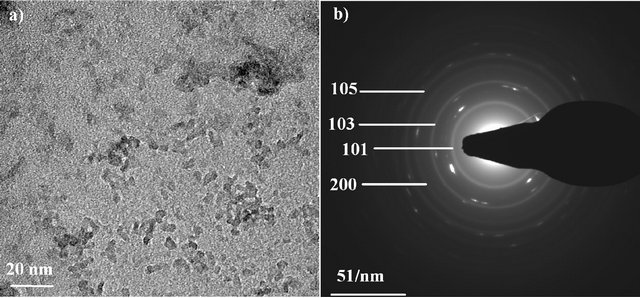
Figure 2. TEM image of TiO2 nanoparticles (a) and SAED pattern of the particles (b).
Table 3. Interplanar distances for the TiO2 nanoparticles deduced from the SAED patterns, compared to the expected ones for ideal anatase phase.

Table 4. Samples characteristics analyzed by TGA.
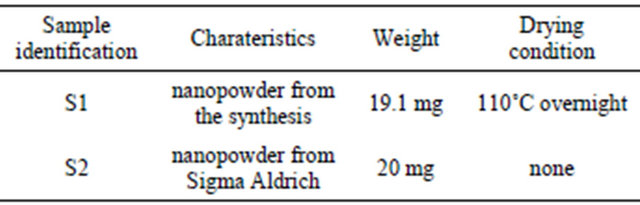
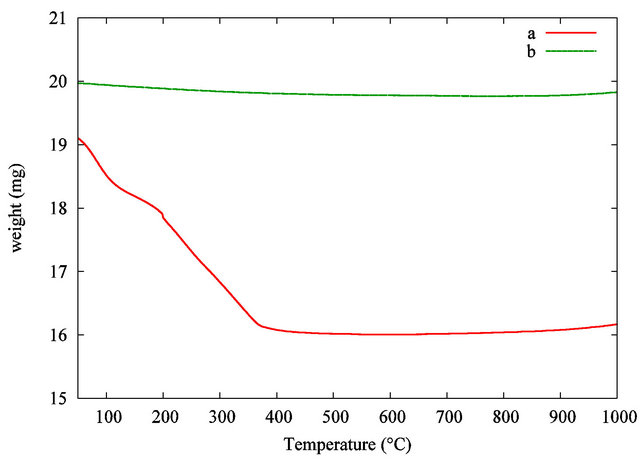
Figure 3. TGA curves of the TiO2 grafted acetylacetone nanopowder from the synthesis (a) and pure TiO2 used as a reference (b).
alcohol. The second weight loss in the 200˚C - 380˚C range is correlated with the progressive thermal degradation of the grafted acetylacetonate group on the TiO2 nanoparticles surface. The pyrolysis of the organic surfactant is taken up to 450˚C. At the highest temperature, in our analytical conditions, no more weight loss is recorded, so pure inorganic TiO2 nanopowder is obtained. A comparison between the samples leads to a total estimated weight loss around 16 wt%. The theoretical suspension concentration is estimated considering the initial amount of precursor and the volume of liquid. This result leads to a calculated estimation of TiO2 concentration in the dispersion around 27 mg·mL−1 or 16400 ppm in titanium. This theoretical result is in good agreement with the experimental value given by TGA.
3.5. Photocatalytic Results
Preliminary, a study of the homogenization of the mixture has been done without irradiation. A volume of 60 µL of the TiO2 suspension was added to 3 mL of AO7 solution (5 × 10−5 mol·L−1). The influence of the stirring time at 200 rpm on the solution homogeneity is summarized in Figure 4. First, by comparison between Figures 4(a) and (b), we evidenced that the addition of TiO2 suspension has a high impact on the AO7 solution’s absorbance at an analytical wavelength of 483 nm. The AO7 solution’s absorbance was decreased nearly to 35% when the TiO2 particles were loaded in the solution. Strong adsorption effect between the organic dye and the nanoparticles may explain this decrease in absorbance. We evidenced that 5 minutes of stirring is required to stabilize the solution’s absorbance. A longer stirring time has no effect when we compare the curves Figures 4(c) (5 minutes of stirring) and (d) (30 minutes of stirring). Accordingly to these preliminaries investigations, all our photocatalytic experiments were carried out after a homogenization step of 5 minutes of stirring at 200 rpm without UV irradiation. The stirring speed remained constant in all the experiments.
The photodegradation behavior of the AO7 solution in presence of nanoparticles was investigated under different conditions summarized in Figure 5.
The AO7 concentration versus the irradiation time was determined by the absorbance’s measurement at the analytical wavelength of 483 nm. Figure 5 curve (a) shows the UV-photodegradation of the AO7 solution (5 × 10−5 mol·L−1) without TiO2 suspension. No significant UV photodegradation is recorded as the solutions concentration remains unchanged during the irradiation. Figure 5
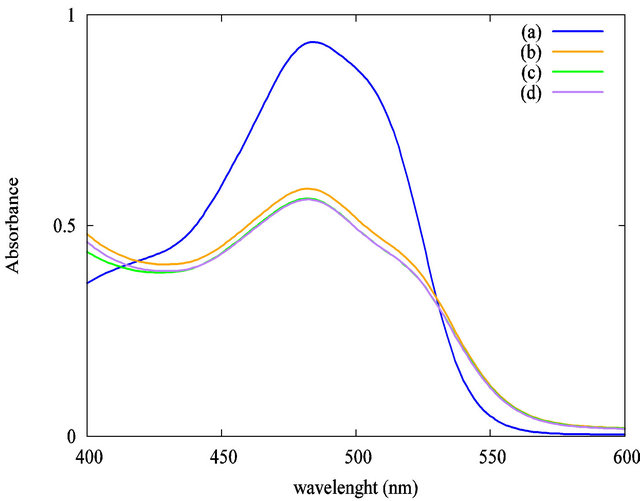
Figure 4. Impact of the stirring time at 200 rpm without UV irradiation on the solution’s absorbance at 483 nm: (a) AO7 only; (b) AO7 and TiO2 suspension without stirring; (c) AO7 and TiO2 suspension, 5 minutes stirring; (d) AO7 and TiO2 suspension, 30 minutes stirring.
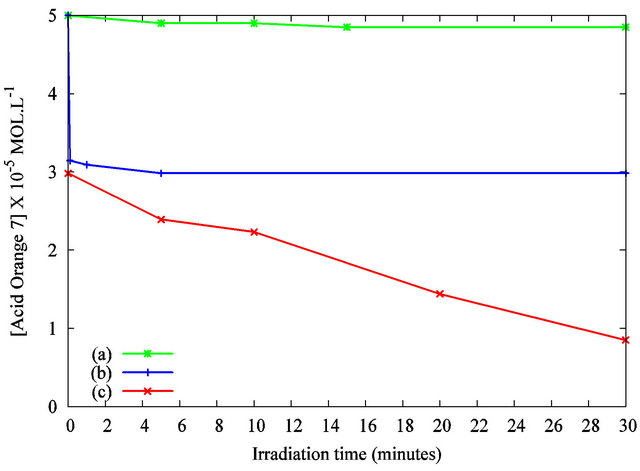
Figure 5. Photodegradation of the Acid Orange 7 solution in the presence of TiO2 nanoparticles under UV irradiation as measured by the solution’s absorbance at λ = 483 nm: (a) AO7 only under UV irradiation; (b) AO7 and TiO2 suspension without UV irradiation; (c) AO7 and TiO2 suspension, under UV irradiation.
curve (b) exhibits that the concentration’s reduction is about 35% without UV curing after 5 minutes of stirring with the designed volume of TiO2 suspension. As previously discussed, the interaction between the organic dye and the particles may explain this decrease. Figure 5 curve (c) highlights the variation in concentration of AO7 with TiO2 nanoparticles under UV irradiation. A quick decrease of the solution’s concentration is evidenced. A complete decolorization of the solution was observed after 30 minutes of irradiation. These results show the photocatalytic activity of the TiO2 nanoparticles dispersed in the organic dye solution. This efficiency is attributed to the anatase crystalline structure of our nanoparticles and to their high specific surface. Compared to previous works published elsewhere [20,21], the photodegradation kinetic is improved by the establishment of a macroscopic liquid/liquid interface compared to a solid/ liquid counterpart. The reproducibility of the results was tested by repeating the measurements. Figure 6 shows the absorbance spectra for 10 minutes of UV irradiation.
The curves concerning the exposure of the AO7-TiO2 mixture with the same UV irradiation time duplicated on two different samples are superimposed, revealing a good reproducibility of the protocol used and validating the method of following the degradation of Acid Orange 7 by the use of UV spectrometry.
3.6. Antibacterial Efficiency Study
Figure 7 shows the effect of our TiO2 nanopowder from the synthesis on Escherichia coli under UV irradiation. Firstly, the result demonstrates that the ultraviolet light used in this experiment did not affect significantly the viability of bacteria after 3 hours in absence of TiO2