1. Introduction
For the transmutation of plutonium and minor actinide elements, various nuclear fuel concepts have been proposed. Uranium-free inert matrix fuel (IMF) is one of the nuclear fuel concepts for plutonium transmutation, socalled “plutonium burning”. Under nuclear reactor operation using uranium-free inert matrix fuel, plutonium will be not newly produced. Also, added fissile plutonium will be almost incinerated [1-5].
An inert matrix nuclear fuel is composed by forming a single or mixed phase. The concept of single phase fuel is based on a fissile material (e.g. plutonium) embedded in the matrix by a solid solution formation. The concept of mixed phase fuel is constituted in the form of CERCER (ceramics embedded in another ceramics, e.g. YyPuxZr1−yO2−y/2-MgAl2O4), CERMET (ceramics embedded in metal, e.g. PuO2-Zr) and METMET (metal phase in a metal matrix, e.g. PuAl4-Al).
Materials for the matrix must satisfy several requirements of the physical properties: high melting point, good thermal conductivity, low solubility in the coolant, good compatibility with the fuel cladding, low neutron cross-section, high density, etc. Various candidates for an inert matrix material have been reviewed [6-10].
Zirconium is one of the elements that satisfy the requirements for inert matrix material [11]. Thus, zirconium has been employed as the inert matrix material, by means of forming a metal [12-14] or oxide [8-10] phase. In particular, zirconium metal matrix based inert matrix fuels can offer a novel approach to fulfill the requirements for “plutonium burning” fuel. In ceramic matrix based IMF fuels, the thermal conductivity is a major concern. In this regard, the thermal conductivity of a zirconium metal matrix is much higher than most ceramic materials.
The thermal conductivity of a material is one of the most important physical properties of the nuclear fuel. In particular, the in-reactor performance, integrity, and safety of inert matrix nuclear fuel are directly affected by the thermal conductivity [15-20]. If the thermal conductivity of nuclear fuel can be improved, the fuel performance will be enhanced in various ways. The lower centerline temperature of a fuel pellet affects the enhancement of fuel safety as well as fuel pellet integrity during nuclear reactor operation. In addition, the nuclear reactor power can be uprated due to a higher safety margin. Therefore, the evaluation of thermal conductivity of inert matrix nuclear fuel is very important as a nuclear fuel.
In the investigation of the zirconium-based inert matrix fuel with plutonium (PuO2-Zr), it has been usually simulated using ZrO2 as a surrogate of PuO2, that is to say, zirconium oxide (ZrO2) ceramic particles were dispersed in a zirconium metal matrix for the safety and convenience in the experiment. In this work, a Zr-ZrO2 simulated inert matrix fuel pellet was fabricated using a hot-pressing method, and several thermophysical properties of the fabricated inert matrix fuel pellet were measured and evaluated.
2. Experimental
Zirconium oxide ceramic particles were used as a surrogate of a plutonium oxide fuel particle. The crystal structures of ZrO2 and PuO2 are the same (a cubic fluorite structure), and similar parameters (milling, sintering, etc.) for both materials can be applied to the fabrication process of fuel pellets containing ZrO2 or PuO2. Therefore, ZrO2 is profitable as a surrogate material for the evaluation of thermal properties.
The physical properties of the surrogated fuel particle (Nikkato, ZrO2) used in this experiment were measured (Table 1). The density and size of the fuel particle were measured using a gas pycnometer (Micromeritics, AccuPyc II) and a particle size and shape analyzer (Sympatec, QICPIC), respectively.
Zirconia particle powder was simply mixed with zirconium metal powder (Sejong materials co., ~30 μm Zr) using a Turbula mixer. Zr-ZrO2 powder mixture was compacted and sintered using a hot pressing method at 800˚C in vacuum and 20 MPa load.
The measured density of the fabricated simulated fuel pellet was 6.008 ± 0.006 g/cc (94.2% of theoretical density). In spite of a low reaction temperature, a highly dense sample could be obtained. Figure 1 shows the zirconia particles homogeneously dispersed in a zirconium metal matrix. It is thought that the thermophysical property of the simulated fuel pellet could be affected by the
Table 1. The measured physical properties of ZrO2 simulated fuel particle which was used as a surrogate.


Figure 1. SEM (Scanning Electron Microscope) image of fracture surface of fabricated fuel pellet.
homogeneity of the particles.
For an evaluation of the thermal conductivity of a simulated inert matrix fuel, several thermophysical properties of simulated fuel pellet were measured. The thermal expansion of simulated fuel was measured using a dilatometer (Netzsch, DIL 402C), and thermal diffusivity of simulated fuel was measured using a laser flash apparatus (Netzsch, LFA-427). Figure 2 shows the simulated inert matrix fuel specimen, which was prepared for thermal diffusivity (about 8 mm diameter) and thermal expansion (about 15 mm length) measurements.
3. Results and Discussion
To evaluate the thermal conductivity of a simulated inert matrix fuel, first of all, the thermal diffusivity of the simulated fuel was measured using a laser flash method. The measured thermal diffusivity of a simulated fuel as a function of temperature is shown in Figure 3. The thermal diffusivity increased with increasing temperature, as a typical tendency of the thermal diffusivity of metal.
The linear thermal expansion as a function of temperature of the simulated fuel was measured using a dilatometry (Figure 4). The measured data was fitted as a function of temperature using a cubic polynomial regression  , where T is an absolute temperature (K) and To is 298 K.
, where T is an absolute temperature (K) and To is 298 K.

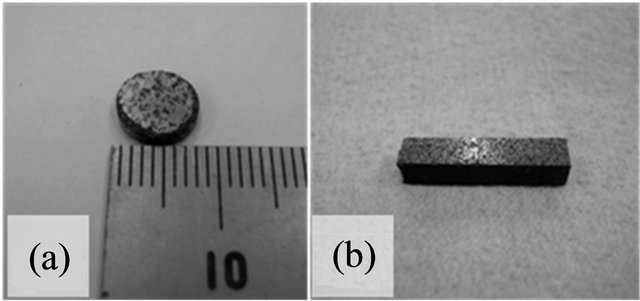
Figure 2. Simulated inert matrix fuel specimen for (a) thermal diffusivity and (b) thermal expansion measurement.
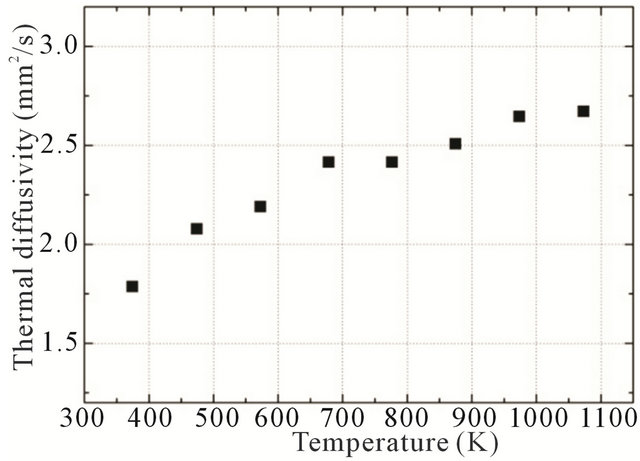
Figure 3. Measured thermal diffusivity as a function of temperature of simulated inert matrix fuel.

Figure 4. Measured linear thermal expansion as a function of temperature of simulated fuel.
The mean coefficients of linear thermal expansion, αp(l), were calculated using the following equation, where L and L298 are the lengths at temperatures of T (K) and 273 K. Also, the calculated linear thermal expansion coefficient of the simulated fuel is shown in Table 2. For comparison, linear thermal expansion coefficients of Zr and ZrO2 were also shown, which were obtained from literature data [21].

Also, based on the literature data and Neumann-Kopp’s rule [22,23], the specific heat of the simulated inert matrix fuel was calculated. Figure 5 shows the calculated specific heat of the simulated fuel.
Finally, the thermal conductivity (kM) of the simulated inert matrix fuel was calculated using the following equation, where DM is the thermal diffusivity, cp is the specific heat, and ρM is the density of the sample as a function of temperature.

For a comparison of thermal conductivity of zirconium and uranium dioxide with the simulated inert matrix fuel, the thermal conductivity of the simulated fuel was normalized to 95% of theoretical density using the following modified-Loeb equation [24],
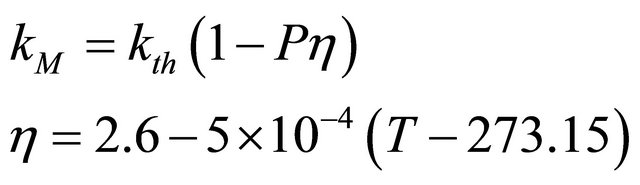
where kth is the thermal conductivity of sample with a density of 100% TD, P is the porosity, and η is the experimental parameter. Also, a thermal conductivity normalized to 95% TD, k95 could be expressed by the following equation.
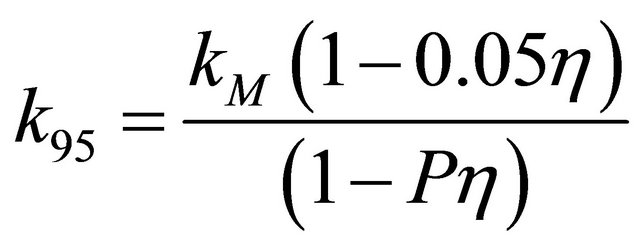
Table 2. The linear thermal expansion coefficient of simulated fuel, based on the measured data.
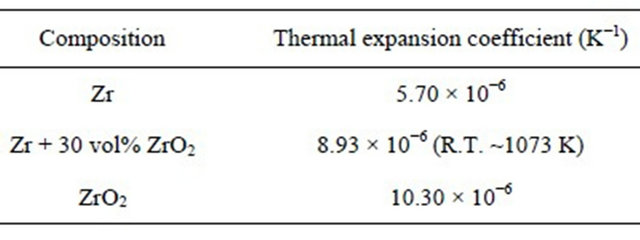

Figure 5. Calculated specific heat of simulated fuel.
The calculated thermal conductivity of the simulated inert matrix fuel based on the thermal diffusivity and thermal expansion data is shown in Figure 6. The thermal conductivity of the fuel increased with an increasing temperature. These results are similar with the typical tendency of the thermal conductivity of Zr metal.
The measured data was fitted as a function of temperature. It was also expressed by the following equation, for 298 K ≤ T ≤ 1073 K.

From the fitted equation, it could be thought that the thermal conductivity of ZrO2-Zr inert matrix fuel was mainly affected by the high thermal conductivity of Zr metal, i.e. an effect of the free electron conduction on the thermal conductivity of the inert matrix fuel was more dominant than that of the phonon conduction.
Figure 7 shows the imperfection of microstructure in the interface between Zr matrix and ZrO2. If the microstructure imperfection in the interface can be entirely improved, the thermal conductivity of ZrO2-Zr inert matrix fuel pellet can be measured more higher.
In the operating temperature (above 700 K) of a LWR (Light Water Reactor) fuel, the thermal conductivity of the inert matrix fuel is higher than that of UO2 (Figure 8).

Figure 6. Thermal conductivity as a function of temperature of simulated inert matrix fuel based on the measured and calculated data.

Figure 7. (a) Imperfect and (b) perfect microstructure in the interface between Zr matrix and ZrO2. SEM image of polished surface.
Therefore, if the inert matrix fuel is used as LWR fuel, it can be thought that the inert matrix fuel is more effective and safe than UO2 as LWR fuel.
4. Conclusions
To evaluate the thermal conductivity of an inert matrix
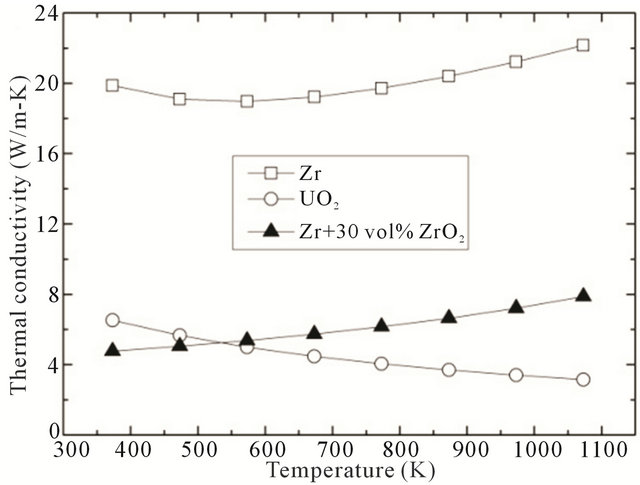
Figure 8. Comparison of thermal conductivity of Zr, UO2 and ZrO2-Zr simulated inert matrix fuel.
fuel, a Zr-ZrO2 simulated inert matrix fuel was fabricated using a hot pressing method, and its thermophysical properties were measured and calculated. The thermal diffusivity and linear thermal expansion of the simulated inert matrix fuel as a function of temperature were measured using a laser flash method and dilatometry, respectively, from room temperature to 1073 K. Also, the specific heat of the simulated fuel was calculated using literature data and Neumann-Kopp’s rule.
In the results, the thermal conductivity of the Zr + 30 vol% ZrO2 simulated inert matrix fuel was 5.75 W/m-K (averaged at temperature range of measurement). It can be thought that the thermal conductivity of Zr-based inert matrix fuel would be dominantly affected by the thermal conductivity of the metal matrix materials.
Also, if metal-based inert matrix fuel is applied to the LWRs as a nuclear fuel, it is expected that the fuel performance and safety can be enhanced, because the high thermal conductivity of metal-based inert matrix fuel leads to the low centerline temperature of fuel.
5. Acknowledgements
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MEST).