Synthesis of Biphasic Calcium Phosphate by Hydrothermal Route and Conversion to Porous Sintered Scaffold ()
1. Introduction
Biphasic calcium phosphate (BCP), particularly hydroxyapatite (HA) and beta tricalcium phosphate (β-TCP) is the one that has been extensively investigated [1-3]. The HA ceramics, despite being considered as the ideal material for bone substitute due to its similarity with the bone mineral and osteoconductive property, it is non-resorbable and bio-inert due to its less soluble nature in aqueous media [4], while β-TCP showed rapid biodegradation [5]. Therefore, a combination of HA with β-TCP will provide osteoconductive material with higher reactivity and better biodegradation.
Various preparation techniques have been developed to produce BCP powders such as powder mixing [6], solid-state reaction [7] and decomposition reaction [8]. In this study, however, a simple hydrothermal route has been used to prepare BCP powder using β-TCP as the precursor which allows controlled phase composition. The β- TCP compound has been reported to be able to completely transform into HA phase under hydrothermal condition [9]. Therefore, biphasic HA/β-TCP could be prepared by incomplete transformation of β-TCP compound under hydrothermal condition.
Natural bone has interconnected porous structure necessary for tissue ingrowth, nutrient supply as well as tissue invasion during bone repairing process [10]. The ideal bone substitute material must possess properties that are close to that of natural bone. A porous ceramics structure particularly composed of biphasic BCP hence, is considered as an ideal material for bone substitute.
2. Materials and Methods
2.1. Synthesis of BCP Powder
To synthesize BCP powder by hydrothermal technique, 5 g of β-TCP powder was dispersed with 500 mL of deionized water. The mixture then was stirred for 1 h in the teflon container to homogenize. After stirring, the Teflon containing mixture then was placed inside the hydrothermal vessel. The hydrothermal condition then was set at 160˚C for 4 h, 24 h, 48 h, and 72 h respectively. Hydrothermal treatment was performed by using SOP HighPressure Autoclave Reactor. After hydrothermal treatment, the as-synthesized HAP was filtered and washed until the pH of the filtered water was neutral, thus removing the acid that was produced in this reaction. The as-synthesized powder was dried in an oven at 90˚C for 24 h. The dried powder was then characterized by X-ray diffraction, XRD (Bruker D2 Phaser), Scanning Electron Microscopy, SEM (Hitachi TM3000 Tabletop Microscope) and Transmission Electron Microscopy, TEM (Philip CM12).
2.2. Fabrication of Porous BCP
To fabricate porous BCP, initially Mg(NO3)2 was dissolved into 30 mL of distilled water and stirred for 10 minutes. Subsequently, 10 g of the synthesized BCP powder was added slowly into Mg(NO3)2 solution to make a slurry. Mg(NO3)2 was added as the sintering aid to densify the porous BCP at relatively lower sintering temperature. A sponge template (polyurethane) was cut into 10 mm × 20 mm × 30 mm in size, and dipped into the slurry. To optimize the impregnation of slurry, the sponge template was squeezed at least ten times whilst dipping. After drying at 60˚C for 2 h, the sponge template with BCP slurry then were sintered in a furnace. The specimens were heated from room temperature to 500˚C at 1˚C/min for 2 h to burn out the sponge template, and then heated to 900˚C at 5˚C/min for 2 h to sinter the ceramics.
3. Results and Discussion
3.1. Characterization of BCP
Figure 1 shows the X-ray diffraction patterns of as-synthesized powder obtained from the hydrothermal treatment. It is observed that β-TCP gradually transformed to HA phase with the longer time under hydrothermal condition. Transformation of β-TCP to HA phase can be explained through the following reaction (Equation (1)) [11]:
Initially, the reaction had transformed around 18 wt% of β-TCP into HA phase after hydrothermally treated for 4 h (calculated by Expert High Score Software). The transformation was indicated from the main peaks of β-TCP at the 2θ of 27.8˚, 31˚ and 34.4˚ which decreased and new peaks, assigned to the HA phase, at the 2θ of 31.7, 32.2 and 32.8 which appeared after hydrothermally treated for 4 h compared to the peaks from the untreated β-TCP powder (Figure 1). The transformation process was continued with longer treating time. The HA phase increased after being hydrothermally treated for 24 h, followed by decreasing the pH of the solution (Table 1). After being treated at 48 h, monetite (CaHPO4) phase was produced besides HA and the remaining β-TCP phases, yielding triphasic powders. Monetite was formed due to the low pH value of the solution as phosphoric acid form, which later reacted with β-TCP. As pH rose, after hydrothermally treated for 72 h, monetite was disappeared. Transformation of β-TCP to CaHPO4 occurred favorably through the following reaction [12]:
 (2)
(2)
Table 1 shows that more than half of the precursor had been transformed into HA and CaHPO4 after being hydrothermally treated for 48 h. After hydrothermally treated for 72 h, monetite had disappeared, yielding only HA and β-TCP phases. The result indicated that monetite had gradually transformed to HA phase with longer treating time. The transformation of monetite to HA phase proceeded through the following reaction (Equation (3)) [13]:

Figure 1. X-ray diffraction of β-TCP powder after being hydrothermally treated.
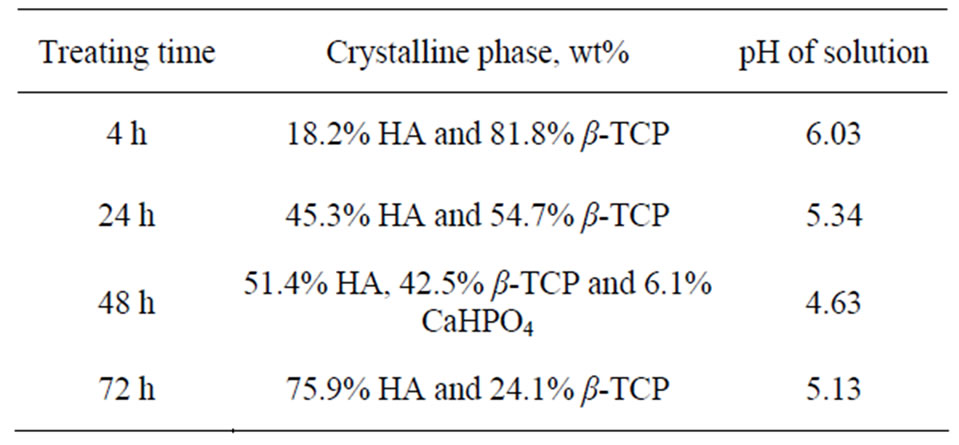
Table 1. Summary of the phase composition and pH of solution of the obtained BCP powder at different treating time.
 (1)
(1)
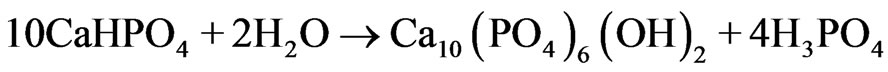 (3)
(3)
Up to this stage, 75.9 wt% of HA phase had been synthesized after 72 h of treating time. The increase of the HA composition in the BCP powder was followed by the decrease of the β-TCP peaks and the increase of the HA peaks in the XRD patterns with longer treating time (Figure 1). β-TCP had been incompletely transformed to HA, yielding biphasic phases of those compounds by the recent hydrothermal treatment. Moreover, gradual transformation of β-TCP to HA during hydrothermal reaction (which is time dependent) allows to control the composition of the BCP powder by manipulating the treating time. Controllable phase composition of BCP is an important factor to manipulate the resorbability of BCP. The resorbability of BCP depends on their HA/β-TCP ratios, with the lower the ratio, the higher the resorbability [14].
Figure 2 shows the change of β-TCP powder morphology after being hydrothermally treated at 160˚C for 72 h. Initially, β-TCP powder was in near spherical shape before hydrothermal treatment (Figure 2(a)). The rodlike shape powder was then obtained after hydrothermal treatment for 72 h (Figure 2(b)), indicating that β-TCP has been transformed into HA powder. However, since β-TCP had remained, spherical powder, which is the β-TCP particles, was also observed. Hydrothermal reaction allows production of HA crystals with larger crystals in acidic condition. In this study, HA particles also tend to grow larger since the pH is in acidic condition as the result of phosphoric acid formation during reaction. Ac-
 (a)
(a)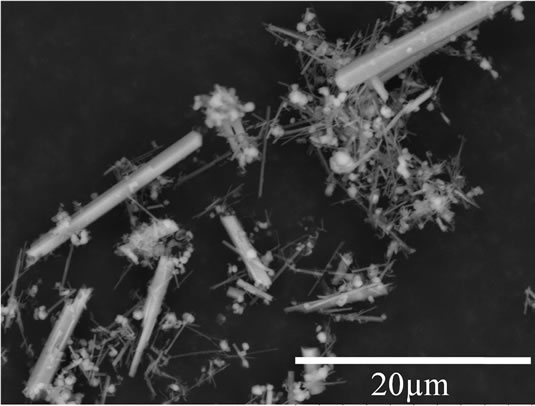 (b)
(b)
Figure 2. SEM images of β-TCP powder (a) and β-TCP after being hydrothermally treated for 72 h (b).
cording to SEM images (Figure 2(d)), it was found that HA particles were intimately mixed with the β-TCP particles, forming a BCP.
The β-TCP particles and as-synthesized particles obtained from the longer treating time (72 h) using TEM are shown in Figure 3. As seen, before being hydrothermally treated, β-TCP was in near spherical and irregular shape particles. These spherical and irregular shape particles then were transformed into rod-like shape HA particles under hydrothermal condition.
3.2. Porous Sintered BCP
The BCP powder obtained from the β-TCP phase transformation under hydrothermal condition was then preceded to porous scaffold by soaking in a template and then sintered. According to XRD patterns (Figure 4), it was observed that the β-TCP peaks were raised after sintering. It indicated that HA has decomposed and reformed into β-TCP phase during sintering.
Figure 5 shows the compositional change after sintering, where β-TCP phase raise from about 24% at the initial pre-sintering to 91% after sintering at 900˚C. Composition of HA phase reduced was due to the result of HA decomposition. Accordingly, HA phase was decomposed at a relatively low sintering temperature, indicated that it exists as calcium deficient HA. The excess of hydrogen (H+) ions in the solution from phosphoric acid were incorporated into HA lattice yielding calcium deficient HA [13]. Calcium deficient HA is decomposed through the following reaction (Equation (4)) [15]: