Physiological Effects of Salmon Milt Nucleoprotein on Movement, Stress Tolerance and Lifespan of C. elegans ()
1. Introduction
Caenorhabditis elegans is a round worm, non-parasitic nematode and lives mainly in soil. It is easy to breed, becomes imago from fertilized eggs through four larval stages in about three days, its lifespan is short at approximately one month, and its semi-transparent body facilitates internal observation. Furthermore, its whole genome has been sequenced and so gene transfer is easy, and the species is frequently used as a human model organism because of the variety of mutants. It is also possible to constantly administer substances when culturing using E. coli as feed, using culture media mixed with some chemical reagents. Various experiments on the administration of substances to nematodes have been reported. For example, increase of stress tolerance by administration of green tea catechin (EGCG) [1], and prolonging of lifespan by addition of NAD [2] and resveratrol [3] have been reported. Studies using glucose overdosage to imitate the obesity model system in higher animals [4], and studies on the relation between unsaturated fatty acid or oxidative stress and physiological function using the RNAi method [5,6], have also been reported.
Salmon milt consists of nucleic acid, protein (mainly protamine) and polyamine. Generally, salmon milt is directly used as a food, and has also long been used as a food additive, such as condiment and preservative. For example, nucleic acid, which is known to show antioxidant and anti-aging effects, has long been used as an additive of powdered milk for infants. Also, amino acid arginine is abundantly contained in protamine, a major ingredient of salmon milt protein, and is known to show various physiological functions such as enhancement of immune response and metabolism, increase of fertility, and activation of insulin secretion [7-9]. Salmon milt extract contains a combination of nucleic acid and nucleoprotein, and is therefore thought to show more effecttive physiological effects. Indeed, effects such as reducetion of tumor, suppression of skin disorder by UV irradiation and improvement of exercise stamina by oral ingestion of salmon milt extract in mouse have been reported [10]. However, although various physiological functions of salmon milt extract are known, their detailed mechanism remains unclear. In this study, we analyzed the physiological functions of salmon milt extract for nematodes and the mechanisms of the functions using the nematode C. elegans as a model organism.
2. Materials and Methods
2.1. Materials
Nucleoprotein prepared from salmon milt was treated by nuclease and protease and was mixed into agar medium for nematode (Nissei Bio Co., Ltd., Hokkaido, Japan).
2.2. Worms
Wild-type Caenorhabditis elegans Bristol N2 (Caenorhabditis Genetics Center, Minnesota, USA) were cultured at 20˚C on NGM (Nematode Growth Medium) agar plated with E. coli OP50 as previously described [11]. Each worm was transferred on the new plate in each 4 days.
2.3. Synchronization of the Growth Stage
To synchronize the growth stage of nematode, 300 adult worms were treated with 10% NaClO solution (10 N NaOH/NaClO (5:1)) at room temperature, until the skin of the individual of 80% was destroyed. The eggs were rinsed by S-basal (0.1 mol/l NaCl, 50 mmol/l potassium phosphate buffer), and the above NaClO treatment was repeated in triplicate. Finally, the collected eggs were cultured overnight in S-basal at 20˚C until the hatching.
2.4. Culture of Worms on Plate Containing NG
NGM plate containing water-soluble salmon milt nuclegen (NG) was prepared for the assay. Escherichia coli strain HT115 transfected with RNAi plasmid L4440 (provided from Fire laboratory) were cultured and were plated on NGM agar containing NG, and were used for culture of nematode. A series of experiments was performed at 20˚C except heat stress tolerance assay.
2.5. Body Size Analysis
The age-synchronized worms were bred on plate containing NG for 72 hr and 96 hr. After the worms had been fixed with PFA (paraformaldehyde, Kanto Chemical, Tokyo, Japan) solution (0.2% PFA/10% EtOH/ S-basal) for 5 min, they were photographed under a microscope (DMRXA, Leica Microsystems, Inc., Bannockburn, IL, USA), and their individual lengths were measured using image analysis software Lia32 (Dr. Kazukiyo Yamamoto, Nagoya University).
2.6. Egg Laying Analysis
The age-synchronized worms were bred on plate containing NG for 72 hr, and were moved to new NGM plate. The number of eggs laid by each worm was counted over a 6 hr period.
2.7. Lifespan Analysis [12]
The age-synchronized worms (20 worms/plate) were bred on plate containing NG (0, 1, 5, 10 mg/ml) plate till they grow up to adult (4th day), then worms were moved to new NG plat. The day when adult was moved was counted as day 0, and the living population was counted every 2 days. Life or death of worms was judged by its response to the dropped S-basal. Worms were moved to new plate every 4 days to prevent the over-growth of E. coli. On day 4, 6 and 8 after the start of culture, worms were treated with 0.5 mg/ml fluorodeoxyuridine (FUdR, Wako, Osaka, Japan) to prevent the next genetical birth.
2.8. The Movement Analysis
The age-synchronized worms were bred plate containing NG for 96 hr (day 0). Then worms were further cultured on plate containing NG for up to 9 days (0, 3, 6, 9 days). Twelve worms were randomly picked and dropped in 20 μl of S-basal on the new plate. The number of movements (pharyngeal pumping and thrashing) per 1 min was counted under the microscope. Worms were moved to new NG plate containing 0.5 mg/ml FUdR (Wako) every 4 days to prevent the over-growth of E. coli.
2.9. Heart Stress Tolerance Assay
The age-synchronized worms were bred plate containing NG for 72 hr. Worms were washed with S-basal buffer, transferred to bacteria-free NGM plates, and incubated at 35˚C for 13 h. After incubation, the worms were immediately cooled at 10˚C for 30 min, and the survival rate of the worms was calculated. Life or death of worms was judged by its response to the dropped S-basal. The worms which showed the viviparous hatching were eliminated.
2.10. Oxidative Stress Tolerance Assay
The age-synchronized worms were bred on plate containing NG for 96 hr, and the worms were transferred to new plates containing NG and 10 mM paraquat (1,1’- Dimethyl-4,4’-bipyridinium Dichloride (TCI Organic Chemicals, Tokyo, Japan)) (day 0). The survival rate was calculated after 6 days. Life or death of worms was judged by its response to dropped S-basal. The worms which showed the viviparous hatching were eliminated.
2.11. Nile Red Assay
The age-synchronized worms were bred on NGM plate containing 50 ng/ml Nile Red (MP Biomedicals, CA, USA) with E. Coli. After 72 hr’ culture, worms were collected, treated with 0.2% PFA solution, and observed under a fluorescence microscope (DMRXA, Leica) with N3 filter (565 nm, Leica) [13].
2.12. RT-PCR and Real Time PCR
The age-synchronized worms (400 worms) were bred plate containing NG for 72 hr, and RNA was extracted by the acid-GTC-phenol method [14]. Genomic DNA was digested by treatment with DNase I (Takara, Otsu, Japan) for 60 min at 37˚C, and RNA was repurified by repeating the acid-GTC-phenol extraction. cDNA was synthesized by using M-MLV Reverse Transcriptase (Takara) and subjected for PCR. The quantity of cDNA was corrected by using gpd-1 (glyceraldehyde 3-phosphate dehydrogenase) as an internal standard. PCR (95˚C for 5 min; 23 - 35 cycles of 95˚C for 1 min, 52˚C for 30 sec, 72˚C for 1.5 min; 72˚C for 7 min) was performed using the specific primers for sod-3 (5’-TGC TGC AAT CTA CTG CTC-3’ and 5’-ATC CTG GTT TGC ACA GGT-3’), sod-4 (5’-TAA TTC TGG CTC TCT CCG-3’ and 5’-GAC GGT ACC GAT AGT TCC-3’), 5’-CAT GGA TCC ATC CAG ATG CAA AGC CAG-3’ and 5’-CAT GGA TCC GTA TGC TGT GCA GCT ACA-3’), gpd-1 (5’-ATG TCG AAG GCC AAC GTC-3’ and 5’-TCG CCA GTG GTG CCA GAC-3’). Real time PCR was performed using the primer for daf-16 (5'-ATC ATC TTT CCG TCC CCG-3’ and 5’-TTG GAA TTG CTG GAA CCG-3’), sod-3 (5’-TAT TAA GCG CGA CTT CGG-3’ and 5’-CTG GTT TGC ACA GGT GGC-3’).
2.13. Statistical Analysis
Results are expressed as mean ± standard deviation. Comparisons between groups were made by analysis of variance (ANOVA), and when significant, were examined by Tukey’s all-pairwise-comparison test. Differences were considered significant when P < 0.05.
3. Results
3.1. NG Affected to Body Size and Reproduction
First, body length, number of laying eggs and lifespan were measured in order to determine whether NG influences the survival rate of nematode. Eggs were treated with NaClO to synchronize their ages, and nematodes at the same developmental stage were used. L1 larval nematodes were bred on a plate containing various concentrations of salmon milt extract (NG), and the body length of the nematodes increased at both 72 hr and 96 hr (Figure 1). Especially, after 72 hr incubation, increase of the body length, depending on the concentration of NG, was observed. The age-synchronized L1 larva was bred on an NG plate for 72 hr, and the obtained young imago (young adult) was used to measure the number of laid eggs every 6 hr (Figure 2). The number of laid eggs was hardly changed by the addition of NG.
3.2. NG Prolonged the Lifespan of Nematode
Furthermore, the age-synchronized L1 larvae were bred on an NG plate for 96 hr (day 0), then the number of living individuals was measured every 2 days. Life and death of the nematodes was judged from reactivity (movement) after dropping of S-basal. As a result, the average lifespan (days of 50% survival rates) was increased by the influence of NG (Figure 3). Especially, with low concentration of NG (1 mg/ml), the prolonging effect was most remarkable and the lifespan was increased by more than 2 days. On the other hand, at high concentrations of NG (5, 10 mg/ml), no remarkable prolonging effect was observed. Concurrently, growth of E. coli, which was used as feed, was remarkably stimulated depending on the NG concentration. Generally, survival rate greatly decreased when the movement of nematodes is inhibited by the excessive E. coli. The increase of E. coli on the high NG concentration plate (5, 10 mg/ml) was so intense that the movement of nematodes was restricted. Incidentally,

Figure 1. NG increased the body size of nematode. The synchronized L1 worms were bred on plate containing NG (0, 1, 5, 10 mg/ml) for 72 hr and 96 hr. Worms were fixed with PFA and their individual lengths were measured using image analysis software. n = 30, error bar indicates standard deviation.

Figure 2. NG had no effect on egg laying. The synchronized L1 worms were bred on plate containing NG (0, 1, 5, 10 mg/ml) for 72 hr, and were moved to new NGM plate. The number of eggs laid by each worm was counted over a 6 hr period. n = 12, error bar indicates standard deviation.

Figure 3. NG prolonged the lifespan of nematode. The synchronized L1 worms (20 worms/plate) were bred on plate containing NG (0, 1, 5, 10 mg/ml) for 96 hr, then worms were moved to new NG plate (day 0). The living population was counted every 2 days. Worms were moved to new plate every 4 days to prevent the over-growth of E. coli. Life or death of worms was judged by its response to the dropped S-basal. n = 165 (NG; 0 mg/ml), n = 143 (NG; 1 mg/ml), n = 118 (NG; 5 mg/ml), n = 122 (NG; 10 mg/ml), error bar indicates standard deviation.
FUdR was added to prevent excessive growth of E. coli in all experiments.
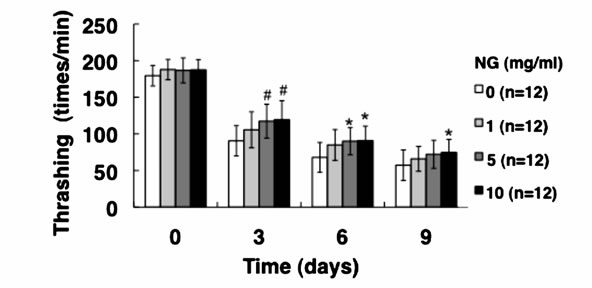
3.3. NG Increased the Movement Activity of Nematode
Generally, mature nematodes grown from parenchymula show gradually decreasing movement as aging progresses [15]. The movement is closely related to the entire lifespan, and individuals which continue to move tend to live longer. Therefore, we analyzed the physiological function of NG for the movement of nematode. Two criteria, gross movement (thrashing) and local movement of pharyngeal shrinkage (pumping), were employed as indications of movement. The age-synchronized L1 larvae were bred on an NG plate for 96 hr (day 0), and movement per minute after a certain period of breeding (0, 3, 6, 9 days) was measured. As a result, the number of movements, both thrashing and pumping, decreased as the days of culture passed. On the other hand, the numbers of both thrashing and pumping movements were increased by the addition of NG depending on the concentration of the NG (Figure 4). Although no significant difference due to individual differences was observed, both local and systemic movements were increased in an NG-concentration dependent manner.
3.4. NG Increased the Stress Tolerance of Nematode
Next, we examined the physiological effects of NG on the stress response of nematode, using two types of stress, heat stress and oxidative stress. The optimal temperature for the survival of nematodes is around 20˚C, and the survival rate decreased to approximately 50% after exposure to the high temperature of 35˚C for 13 hr (Figure 5(a)).
 (a)
(a)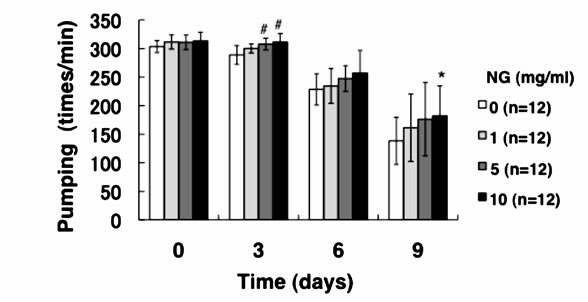 (b)
(b)
Figure 4. NG increased the movement activity of nematode. The synchronized L1 worms were bred on plate containing NG (0, 1, 5, 10 mg/ml) for 96 hr (day 0). Then worms were further cultured on plate containing NG for 0, 3, 6, 9 days. Twelve worms were randomly picked and dropped in 20 ml of S-basal on the new plate. The number of thrashing (a) and pharyngeal pumping (b) per 1 min was counted under the microscope. Worms were moved to new NG plate containing 0.5 mg/ml FUdR (Wako) every 4 days to prevent the over-growth of E. coli. n = 12, error bar indicates standard deviation.
In contrast, the survival rate of nematodes bred in the presence of NG under the same heat stress increased to more than 70%. Furthermore, nematodes were bred on an NG plate containing paraquat as an oxidative stress source. When paraquat is incorporated into the body, reactive oxygen species are produced. Nematodes that had been bred on the NG (+/–) plate 96 hr earlier were subsequently bred on the NG (+/–) plate containing 10 mM paraquat. As a result, survival rates of approximately 75% in the presence of low NG concentration (1 mg/ml) and 90% in the presence of high NG concentration (5, 10 mg/ml) were observed, whereas the survival rate was approximately 45% in the case of NG (–) (Figure 5(b)). Therefore, NG was proven to remarkably increase the tolerance of nematodes to heat stress and oxidative stress.
3.5. The Effect of NG on Fat Accumulation
Next, we analyzed the change of adiposity by using the Nile Red method. Red fluorescence is developed by Nile Red staining when lipid droplets exist, and intense fluorescence develops as the amount of the lipid droplet
 (a)
(a)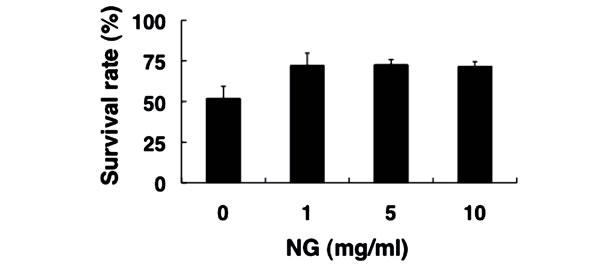 (b)
(b)
Figure 5. NG increased the stress tolerance of nematode. (a) The synchronized L1 worms were bred plate containing NG (0, 1, 5, 10 mg/ml) for 72 hr. Worms were washed with S-basal buffer, transferred to bacteria-free plates, and incubated at 35˚C for 13 h. After incubation, the worms were immediately cooled at 10˚C, and the survival rate was calculated; (b) The synchronized L1 worms were bred on plate containing NG (0, 1, 5, 10 mg/ml) for 96 hr, and the worms were transferred to new NG plates containing 10 mM paraquat (day 0). The survival rate was calculated after 6 days. Life or death of worms was judged by its response to dropped S-basal. The worms which showed the viviparous hatching were eliminated. n = 3, Error bar indicates standard deviation.
is increased. In the case of 1 and 5 mg/ml NG concentrations, fluorescence after the 150 synchronized individuals were cultured in culture media containing Nile Red for 72 hr was stronger than that of NG (–). On the other hand, in the presence of 10 mg/ml NG, fluorescence was hardly observed (Figure 6). Therefore, NG was proven to influence the amount of fat accumulation in the nematode body. However, according to recent studies, the Nile Red method does not stain triglyceride (TG) directly.
3.6. NG Increased the Gene Expression of Sod
Generally, daf-16, which is known as a longevity gene, is considered to be closely related to fatty acid metabolism and adiposity. A previous study showed that the expression level of daf-16 in individuals which presented intense fluorescence by Nile Red staining was increased [16]. Therefore, we analyzed gene expression by the RTPCR method. RNA was extracted from individuals bred on the NG plate for 72 hr, and the expression levels of daf-16, sod-3 and sod-4 gene were measured by the RTPCR method. As a result, the expression level of sod-3 was

Figure 6. The effect of NG on fat accumulation. The synchronized L1 worms were bred on plate containing 50 ng/ml Nile Red with E. Coli. After 72 hr’ culture, worms were collected, treated with 0.2% PFA solution, and observed under a fluorescence microscope with N3 filter.
increased after breeding on the NG (+) plate (Figure 7), and the expression level of sod-4 was increased by NG (1 mg/ml), whereas the expression of daf-16 was changed only a little. Quantitative PCR verified the similar tendency of mRNA expression of daf-16 and sod-3 (Figure 7(b)). Because the activity of transcription factor Daf-16 is affected by protein modifications such as phosphorylation and acetylation, it is currently analyzed at the protein level.
4. Discussion
The results of this study showed that the average lifespan (days showing 50% survival rates) of the nematodes was prolonged by the addition of NG (Figure 3). Especially, in the case of a low concentration of NG (1 mg/ml), the prolonging effect was most remarkable and the lifespan was extended by more than 2 days. Generally, nematodes bred under caloric restriction and variants with low mobility show prolonged individual lifespans [17]. Thus, the lifespan under caloric restriction is thought to be prolonged through the activation of Sir2.1 by an increase of NAD [2]. However, the amount of feed in this study was abundant and the nematodes took sufficient calories. Considering the number of times of gross movement (thrashing), movement was restored by the addition of NG (Figure 4(a)). Further, in an experiment of pharyngeal pumping, calorie intake with NG supplied was higher than that of the control group (Figure 4(b)). However, the longer lifespan induced by intake of NG (Figure 3) suggests that NG affects a different pathway from that affected by caloric restriction. In addition, lifespan was not remarkably prolonged under a high concentration of NG (5, 10 mg/ml) (Figure 3). The reason is thought to be an inhibition of movement of nematodes due to increase of E. coli used for feeding after addition of NG, leading to a decreased survival rate. The body length was increased by the addition of NG in a con-
 (A)
(A) (B)
(B)
Figure 7. NG affected to gene expression. The synchronized L1 worms (400 worms) were bred plate containing NG for 72 hr, and RNA was prepared for RT-PCR. (A) The quantity of cDNA was corrected by using gpd-1 as an internal standard. PCR (95˚C for 5 min; 23 - 35 cycles of 95˚C for 1 min, 52˚C for 30 sec, 72˚C for 1.5 min; 72˚C for 7 min) was performed using the each primer for daf-16, sod-3 and sod-4; (B) The mRNA level of each gene was analyzed by real-time PCR. PCR (95˚C for 15 s, 60˚C for 1 min, for 40 cycles) was performed using the specific primers for daf-16 (a) and sod-3 (b).
centration-dependent manner (Figure 1). Furthermore, the number of laid eggs was not influenced by NG (Figure 2), and adiposity varied with the NG concentration (Figure 6). Generally, prolonging of lifespan and fatty acid metabolism or adiposity are known to be closely associated with transcription factor daf-16 [2,16]. Although the molecular mechanism of NG activity is unknown, the finding of this study that the amount of fat accumulation and body length vary with NG concentration is interesting.
In this study, both thrashing and pumping movement decreased as the breeding days passed (Figure 4). This means that movement decreased with aging of nematode. However, in the presence of NG, the number of both movements was increased depending on the addition concentration of NG (Figure 4). Therefore, it is suggested that NG is effective for restoring lost mobility with individual aging. Further, a remarkable increase of survival rate of nematodes bred in the presence of NG under heat stress is particularly interesting (Figure 5(a)). Also, although the survival rate of nematodes is greatly decreased by active oxygen stimulation, by addition of NG, the survival rate was increased (Figure 5(b)). These results indicate that the tolerance of nematodes to heat stress and oxidative stress were remarkably increased by NG.
According to gene expression analysis, the expression of sod-3 [18] and sod-4 was increased by addition of NG (Figure 7). sod-3 and sod-4 are scavenger genes involved in the removal of active oxygen, therefore the longer lifespan and greater stress tolerance induced by NG may be due to the increase of these sod gene expressions. Generally, sod-3 is controlled by transcription factor daf- 16 [19], which is known as a longevity gene and is related to longer lifespan [20], adiposity [16], and stress tolerance [21]. Because NG extends lifespan and promotes stress tolerance, NG may be involved to the increase of sod-3 expression and resultant reduction of ROS level through daf-16. However, RT-PCR showed that the expression of mRNA of daf-16 was hardly influenced directly by NG (Figure 7). Meanwhile, sod-4 is also a stress resistance gene, but is controlled by pha-4, not daf-16 [22]. In addition, SKN-1, a transcription factor which controls the expression of ROS-scavenging genes might be also involved in the physiological function evoked by NG [23]. The activity of these transcription factors, including daf-16, may be changed through protein modifications such as phosphorylation and acetylation, as well as changes of RNA expression level. Further detailed analysis of protein, as well as RNA level analysis, including the contribution of transcription factors other than daf-16, are necessary in future. Also, NG shows an effect of restoring movement lost with aging. The degree of influence on increasing the expression level of stress resistance genes, such as sod, on movement, as well as its detailed mechanisms, remains unclear. Factors other than sod, relating to stress tolerance, e.g. heat shock protein, may be involved. Factors relating to movement such as AMPK require a more detailed analysis.
5. Conclusion
In this study, we analyzed the physiological functions of salmon milt extract for nematodes and the mechanisms of the functions using the nematode C. elegans as a model organism. As the results, salmon milt extract increased average lifespan of nematode, and restored the decreased movement with aging. Furthermore, salmon milt extract promoted stress tolerance against heat and oxidative stress by upregulating the expression of stress-tolerance-related genes. These results suggested that salmon milt nucleoprotein possess multiple physiological effects in model animal, and show the potential availability as useful materials for human health.
6. Acknowledgements
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan.
NOTES