Arsenic Removal from Drinking Water by Self-Made PMIA Nanofiltration Membrane ()
1. Introduction
Arsenic is one of several hazardous inorganic species that seriously threaten aquatic environments. Chronic arsenic intake can cause various health effects including skin lesions such as hyperkeratosis and pigmentation changes, circulatory disorders, diabetes and cancers of the bladder, lungs and skin [1,2]. Consequently, a new maximum contaminant level (MCL) standard of 10 μg/L arsenic in drinking water recommended by the WHO has been accepted both within the European Union and by the EPA in the USA [3-5].
These new regulations impose a demand for more efficient arsenic removal from drinking water. Among conventional arsenic removal technologies mainly utilizing adsorption and coagulation processes, membrane processes have emerged as a promising new route for high quality water purification. These approaches have many advantages, including no requirement for the addition of chemical substances, easy increase of capacity, separation in the continuous mode and the possibility to easily join membrane processes with other unit processes [6-9]. Arsenic removal pressure-driven membranes are mainly based on reverse osmosis (RO), nanofiltration (NF), ultrafiltration (UF) or microfiltration (MF) [10-12]. Of the available membrane technologies applicable as clean-up methods for drinking water, NF is efficiently able to remove arsenic from water due to the small size of the membrane pores between RO and UF. Because of its advantages, such as low operating pressure and high retention of multivalent anions, it has generated worldwide interest and has been utilized in the removal of various pollutants from drinking-water [13-15]. Recent developments have proved that NF to be a potential technology for arsenic removal from drinking water charging different experimental parameters [16-18].
However, arsenic removal depends on many factors such as membrane properties, feed water composition, pH and the presence of other co-existent ions. Further investigations changing these parameters are of interest for the development of an arsenic removal strategy utilizing a NF process.
In the present study, a novel self-made PMIA NF membrane was used to remove arsenic by NF. The performance was examined with relation to arsenic rejection under changing operating conditions.
2. Experimental
2.1. Materials
Na2HAsO4·7H2O salt (Merck 6284) was dissolved in deionized water to produce a 1 mg/L As(V) stock solution. This stock solution was diluted to prepare As(V) in the experimental range. pH was adjusted with either HCl or NaOH solution. Poly (m-phenylene isophthalamide) (PMIA) (shown in Figure 1, from Yantai Spandex Co. Ltd. China), N, N’-dimethylacetamide (DMAC), LiCl and acetone (analytical grade, Tianjin Fuchen Chemicals Reagent Factory, China) were used in the preparation of the NF membrane. Different salts (Na2SO4, NaCl) and other chemicals used in the experiments were all of analytical grade without further purification. A PMIA nanofiltration membrane was self-prepared in our laboratory.
2.2. NF Membrane Characterization
The morphology of the PMIA self-made membrane was analyzed using a scanning electron microscope (SEM) (JSM-6301F, JEOL). The samples were cryogenically fractured in liquid nitrogen and sputter-coated with a thin gold film prior to SEM observation.
The zeta potentials of the membrane were determined through electrophoretic mobility measurements in 500 mg/L KCl solution background electrolyte at various pH values. From the measured electrophoretic mobility, the zeta potential was using calculated [15,16].
2.3. Arsenic Removal by NF Membrane
A schematic diagram of the NF experimental setup is shown in Figure 2. The NF membrane module was an annular chamber made of stainless steel in which a membrane supported by porous sintered steel was mounted. The effective area of the membrane was 21.2 cm2. Each new membrane was initially operated for 30 min at 1.0 MPa before commencing the actual separation study.
The effect of different operating conditions such as

Figure 2. Schematic diagram of the experimental setup.1: feed tank; 2: precision filter; 3: pump; 4: membrane module; 5: permeate; 6: flowmeter; 7: heater; 8, 9 and 10: valve; 11 and 12: pressure gauge.
membrane properties, As feed concentration, operating pressure, pH and co-existent ions on As rejection was investigated in experimental trials. Each experiment was repeated at least three times to obtain the results presented in this study, with results averaged with a variation of ±5%.
The rejection R, defined as:
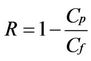 (1)
(1)
with Cp and Cf as permeate and feed concentration, respectively, was determined in each experiment.
2.4. Sample Analyses
The arsenic concentration was analyzed on an atomic fluorescence spectrometer (AF-610A, Rayleigh Analytical Instrument Co., China). In order to determine reproducibility of results, each group of experiments was repeated. The experiments were performed in duplicate and mean values considered. The blank controls showed no detectable arsenic or iron adsorbed onto the flask walls. The pH value of the solutions was measured with a pH meter (FE20, Mettler Toledo).
3. Results and Discussions
3.1. NF Membrane Structure and Performance
The SEM morphology of our self-made membrane is shown in Figure 3. From Figure 3(a), we can see that surface of the self-made NF membrane has a dense and tight structure. From Figure 3(b) of the cross-section of the NF membrane, we can see that the originally porous surface of substrate is covered by a flat featureless PMIA layer.
The zeta potential of the membrane as a function of pH is shown in Figure 4. The self-made membrane was always negative within the experimental pH ranges from 3 to 9. In general, the membrane became more negative with increasing pH.
3.2. Effect of Arsenic Concentration on Arsenic Removal
Figure 5 shows the effect of As feed concentration on As(V) rejection for NF membrane at 25˚C and 0.5 MPa. As(V) rejection was higher than 90% in the range of investigated As feed concentrations. As concentration in the permeate increased with increasing feed As concentration. As detected in the permeate was lower than the EPA-recommended MCL up to a feed As concentration of about 10 μg/L.
3.3. Effect of Operating Pressure on Arsenic Removal
In order to examine the influence of operating pressure
 (a)
(a) (b)
(b)
Figure 3. SEM photographs of the self-made membrane (a) Surface 10,000×; (b) Cross-section 2000×.
on the removal of arsenic species, experiments were investigated at a feed As concentration of 60 μg/L at 25˚C and pH = 7, results are shown in Figure 6. It can be seen that As(V) rejection was higher than 90% over the pressure range investigated. In general, arsenic removal efficiency increased slightly with increasing pressure. This can be attributed to an increase in permeate flux with increasing pressure, resulting in lower arsenic concentration in the permeate and subsequently improving As(V) rejection [9,19].
The concentration of As(V) in the permeate decreased with increasing pressure. The amount of As detected in the permeate was lower than the EPA recommended MCL up to a feed As concentration of about 10 μg/L over the pressure range investigated.
3.4. Effect of pH on Arsenic Removal
Removal of arsenic by NF membrane is influenced by membrane surface charge (represented by the zeta potential) [9]. pH is one of the most important parameters controlling As(V) species from water body. For pentavalent arsenic in a pH range of 2.0 - 7.0, the corresponding stable species is ; above pH 7.0, the corresponding stable species is
; above pH 7.0, the corresponding stable species is  [14]. To examine the influ-
[14]. To examine the influ-

Figure 4. Streaming potential of membrane at different pH.

Figure 5. Effect of As feed concentration on the removal of As(V) (temperature = 25˚C, pH = 7, pressure = 0.5 MPa).
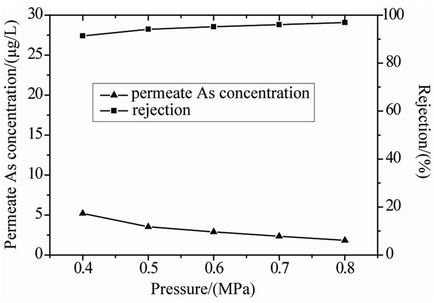
Figure 6. Effect of operating pressure on the removal of As(V) (feed concentration = 60 μg/L, temperature = 25˚C, pH = 7).
ence of pH on the removal of arsenic species, experiments were investigated in a pH range 3 to 11 at a feed As concentration of 60 μg/L, 25˚C and 0.5 MPa, and results are shown in Figure 7.
It can be seen that As(V) rejection increased significantly with increasing pH. Specifically, As(V) rejection

Figure 7. Effect of the pH on the removal of As(V) (feed concentration = 60 μg/L, temperature = 25˚C, pressure = 0.5 MPa).
increased from 83% at pH 3 to 99% at pH 9. The increase in As(V) rejection at higher pH can be attributed to the two factors. Firstly, the magnitude of electric repulsion on divalent As(V) would be higher than that on monovalent As(V); the monovalent ion ( ) is dominant in a pH range of 3 - 6 whist the divalent ion (
) is dominant in a pH range of 3 - 6 whist the divalent ion (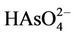 ) is dominant above pH 7. Divalent ions are rejected at a much higher rate compared to monovalent ones due to the larger hydrated radii of divalent ions. Secondly, the membrane charge density in terms of zeta potential decreases with pH increase; membrane becomes more negative as pH increases, increasing the effect of charge exclusion in rejection. This is consistent with the previous observation that NF membranes exhibit a typical Donnan exclusion behavior and charge interaction charge the separation of negative solutes [20,21]. This result indicates that pH has a significant influence on As(V) removal due to arsenic speciation with variation in pH [15,22].
) is dominant above pH 7. Divalent ions are rejected at a much higher rate compared to monovalent ones due to the larger hydrated radii of divalent ions. Secondly, the membrane charge density in terms of zeta potential decreases with pH increase; membrane becomes more negative as pH increases, increasing the effect of charge exclusion in rejection. This is consistent with the previous observation that NF membranes exhibit a typical Donnan exclusion behavior and charge interaction charge the separation of negative solutes [20,21]. This result indicates that pH has a significant influence on As(V) removal due to arsenic speciation with variation in pH [15,22].
The concentration of As(V) in the permeate decreased in the range of the investigated pH. As detected in the permeate was below than the EPA recommended MCL up to a feed As concentration of about 10 μg/L. For membrane the highest arsenic removal corresponds to the lowest arsenic concentration in permeate occurred at higher pH.
3.5. Effect of Other Ions on Arsenic Removal
Various inorganic substances exist in natural waters, and the presence of these substances can interfere with arsenic removal, so it is important to interpret the rejection characteristics of arsenic species in a mixed salt solution using a NF process. NaCl and Na2SO4 were selected to assess the effects of co-existing anions on arsenic removal.
Figure 8 shows the removal of As from DI water, 10 mN NaCl and 10 mN Na2SO4 solutions at pH 7 by our self-made membrane. Consistent with Donnan exclusion,